Iron is transported and stored in the body through a sophisticated system involving specialized proteins, and at worldtransport.net, we aim to clarify this process for you. This system ensures that iron, crucial for various bodily functions, is efficiently delivered to cells and safely stored to prevent toxicity; hence, maintaining optimal iron levels is vital for overall health. Dive in for innovative strategies and up-to-date information about iron metabolism, iron homeostasis, and cellular iron handling.
1. What Is the Primary Mechanism for Iron Transport in the Blood?
The primary mechanism for iron transport in the blood involves transferrin, a protein specifically designed to bind and carry iron. Transferrin ensures iron, vital for oxygen transport and enzyme function, reaches cells safely and efficiently.
1.1. The Role of Transferrin in Iron Transport
Transferrin plays a critical role by binding to iron ions (specifically Fe3+, the ferric form) and transporting them through the bloodstream to various tissues and organs. This process is essential because free iron ions can be toxic, catalyzing the formation of harmful free radicals. According to research from the National Institutes of Health (NIH) in July 2023, transferrin ensures iron is delivered only to cells with transferrin receptors, preventing oxidative damage.
1.2. Oxidation of Iron: The Role of Hephaestin and Ceruloplasmin
For iron to bind to transferrin, it must be in the Fe3+ state. Iron absorbed from the diet is often in the Fe2+ (ferrous) form. Two copper-containing proteins, hephaestin and ceruloplasmin, play key roles in oxidizing Fe2+ to Fe3+.
- Hephaestin: This protein is located in the membrane of enterocytes (intestinal cells) and works in conjunction with ferroportin, the protein responsible for transporting iron out of the enterocytes into the bloodstream. Hephaestin oxidizes Fe2+ to Fe3+ as it exits the cell, allowing it to bind to transferrin.
- Ceruloplasmin: This is the major copper transport protein in the blood. While hephaestin primarily functions in the intestines, ceruloplasmin also helps oxidize Fe2+ to Fe3+ in other tissues, especially when iron levels are low, according to a study published in the “American Journal of Clinical Nutrition” in February 2024.
1.3. Transferrin Receptors and Cellular Iron Uptake
Once Fe3+ is bound to transferrin, the transferrin-iron complex travels through the blood until it encounters cells with transferrin receptors on their surface. These receptors are found on nearly all cells, but are particularly abundant on cells with high iron requirements, such as red blood cell precursors in the bone marrow.
- Binding: Transferrin binds to the transferrin receptor on the cell surface.
- Endocytosis: The transferrin-receptor complex is then internalized into the cell via endocytosis, forming a vesicle.
- Iron Release: Inside the vesicle, the environment is acidified, which causes the iron to be released from transferrin. The Fe3+ is converted back to Fe2+, which is then transported out of the vesicle into the cytoplasm via the divalent metal transporter 1 (DMT1).
- Receptor Recycling: The transferrin and its receptor are then recycled back to the cell surface, where transferrin is released back into the bloodstream to pick up more iron.
2. What Are the Primary Iron Storage Proteins in the Body?
The primary iron storage proteins in the body are ferritin and hemosiderin, which prevent iron’s toxic effects by storing it safely. Ferritin is the main storage protein, while hemosiderin stores iron when levels are high.
2.1. Ferritin: The Major Iron Storage Protein
Ferritin is the primary intracellular iron storage protein. It can store iron in a soluble, non-toxic form, making it readily available when needed. Ferritin consists of a protein shell (apoferritin) surrounding an iron core that can contain up to 4,500 iron atoms.
- Location: Ferritin is found in most tissues, but is particularly concentrated in the liver, spleen, and bone marrow – the major sites of iron storage.
- Function: When iron levels are high, iron is stored as ferritin. When iron levels are low, iron is released from ferritin to be used for various cellular processes or transported to other parts of the body via transferrin.
- Clinical Significance: Serum ferritin levels are often used as an indicator of the body’s iron stores. Low ferritin levels typically indicate iron deficiency, while high levels can indicate iron overload or inflammatory conditions.
2.2. Hemosiderin: Long-Term Iron Storage
Hemosiderin is another iron storage protein, but it stores iron in a less accessible form than ferritin. It is a partially degraded form of ferritin and is typically found in tissues when there is iron overload.
- Location: Hemosiderin is found primarily in the liver, spleen, and bone marrow, especially when iron levels are high.
- Function: Hemosiderin accumulates when the body has more iron than can be stored in ferritin. It is a long-term storage form of iron, but the iron is not as readily available as that stored in ferritin.
- Clinical Significance: Hemosiderin deposits can be seen in conditions such as hemochromatosis (iron overload disorder) and in cases of chronic bleeding or transfusions.
2.3. Comparison of Ferritin and Hemosiderin
To better understand the differences between ferritin and hemosiderin, here’s a comparative table:
| Feature | Ferritin | Hemosiderin |
|---|---|---|
| Primary Role | Short-term, readily available iron storage | Long-term, less accessible iron storage |
| Structure | Protein shell with iron core | Partially degraded ferritin aggregates |
| Availability | Iron readily released when needed | Iron released more slowly |
| Storage Level | Normal iron levels | Iron overload conditions |
| Clinical Use | Indicator of iron stores in the body | Indicator of iron overload in tissues |
3. What Are the Three Major Compartments of Iron in the Body?
The three major compartments of iron in the body are functional iron, storage iron, and transport iron. Functional iron is used in essential processes, storage iron is reserved for future needs, and transport iron moves iron between tissues.
3.1. Functional Iron: Hemoglobin, Myoglobin, and Iron-Containing Enzymes
Functional iron refers to iron that is actively involved in various physiological processes. This compartment can be further divided into three subcompartments:
- Hemoglobin: Found in red blood cells, hemoglobin is responsible for transporting oxygen from the lungs to the tissues. Each hemoglobin molecule contains four iron atoms, each capable of binding one molecule of oxygen. Hemoglobin accounts for the largest portion of functional iron in the body.
- Myoglobin: Found in muscle cells, myoglobin stores oxygen and releases it when needed for muscle contraction. Similar to hemoglobin, myoglobin contains one iron atom per molecule.
- Iron-Containing Enzymes: Iron is a cofactor for many enzymes involved in essential metabolic pathways, such as energy production, DNA synthesis, and antioxidant defense. Examples include cytochromes, iron-sulfur cluster enzymes, and catalase.
3.2. Storage Iron: Ferritin and Hemosiderin
Storage iron includes iron stored in ferritin and hemosiderin, primarily in the liver, spleen, and bone marrow. This iron serves as a reserve that can be mobilized when the body’s iron requirements increase or when dietary intake is insufficient.
- Ferritin: As mentioned earlier, ferritin is the primary iron storage protein, capable of storing large amounts of iron in a readily available form.
- Hemosiderin: Hemosiderin is a long-term storage form of iron, typically found in tissues when there is iron overload.
3.3. Transport Iron: Transferrin
Transport iron refers to iron that is bound to transferrin in the bloodstream. Transferrin delivers iron to cells throughout the body, ensuring that iron is available for functional and storage purposes.
3.4. Distribution of Iron Among the Compartments
The distribution of iron among the different compartments varies depending on factors such as age, sex, and overall health. The following table provides an overview of iron distribution in adults:
| Compartment | Men (mg Fe/kg body weight) | Women (mg Fe/kg body weight) |
|---|---|---|
| Functional Iron | ||
| Hemoglobin | 32 | 28 |
| Myoglobin | 5 | 4 |
| Fe-containing Enzymes | 1-2 | 1-2 |
| Storage Iron | ||
| Ferritin & Hemosiderin | ~11 | ~6 |
| Transport Iron | ||
| Transferrin | 0.04 | 0.04 |
As the table shows, the majority of iron in the body is found in the functional iron compartment, particularly in hemoglobin. Storage iron accounts for a significant portion, while transport iron represents a relatively small fraction.
4. Why Is Iron Recycling Important in the Body?
Iron recycling is crucial because the body has limited ability to excrete iron, making reuse of iron from old red blood cells essential for maintaining iron balance. This process conserves iron and reduces the need for dietary intake.
4.1. The Lifespan of Red Blood Cells
Red blood cells have a lifespan of approximately 120 days. After this time, they are broken down in the liver, spleen, and bone marrow. The iron released from hemoglobin during this process is recycled and reused.
4.2. The Process of Iron Recycling
When red blood cells are broken down, hemoglobin is degraded, and iron is released. This iron can then be:
- Used for Cellular Purposes: Iron can be used directly by cells for various functions, such as acting as a cofactor for enzymes.
- Stored in Ferritin or Hemosiderin: Iron can be stored in the liver, spleen, and bone marrow as ferritin or hemosiderin for later use.
- Transported to Other Tissues on Transferrin: Iron can be bound to transferrin and transported to other tissues where it is needed, such as the bone marrow for new red blood cell synthesis.
4.3. Efficiency of Iron Recycling
Iron recycling is a highly efficient process. According to the National Institutes of Health (NIH), the body recycles approximately 20-30 mg of iron per day, which is significantly more than the amount obtained from dietary intake (1-2 mg per day).
4.4. Impact on Iron Homeostasis
Efficient iron recycling helps maintain iron homeostasis, ensuring that the body has an adequate supply of iron without relying solely on dietary intake. This is particularly important because the body has limited ability to excrete excess iron.
5. How Does the Body Regulate Iron Absorption?
The body regulates iron absorption primarily through the hormone hepcidin, which controls the release of iron from cells into the bloodstream. Hepcidin ensures that iron absorption is adjusted based on the body’s iron levels and needs.
5.1. The Role of Hepcidin
Hepcidin is a peptide hormone produced by the liver. It plays a central role in regulating iron homeostasis by controlling the release of iron from enterocytes (intestinal cells) and macrophages (immune cells that recycle iron from red blood cells) into the bloodstream.
5.2. Mechanism of Action
Hepcidin acts by binding to ferroportin, the only known iron exporter protein. When hepcidin binds to ferroportin, it causes ferroportin to be internalized and degraded. This prevents iron from being transported out of the cell and into the bloodstream.
5.3. Regulation of Hepcidin Production
Hepcidin production is regulated by several factors, including:
- Iron Levels: High iron levels increase hepcidin production, while low iron levels decrease hepcidin production. This ensures that iron absorption is reduced when iron stores are high and increased when iron stores are low.
- Inflammation: Inflammatory cytokines, such as interleukin-6 (IL-6), increase hepcidin production. This is why chronic inflammation can lead to anemia of chronic disease, where iron is trapped in cells and unavailable for red blood cell production.
- Erythropoietic Activity: Increased erythropoietic activity (red blood cell production) decreases hepcidin production. This ensures that more iron is available for red blood cell synthesis when the body needs more red blood cells.
5.4. Clinical Significance
Dysregulation of hepcidin production can lead to various iron-related disorders. For example, in hemochromatosis, a genetic disorder characterized by iron overload, hepcidin production is inappropriately low, leading to excessive iron absorption. Conversely, in anemia of chronic disease, hepcidin production is inappropriately high, leading to iron deficiency anemia.
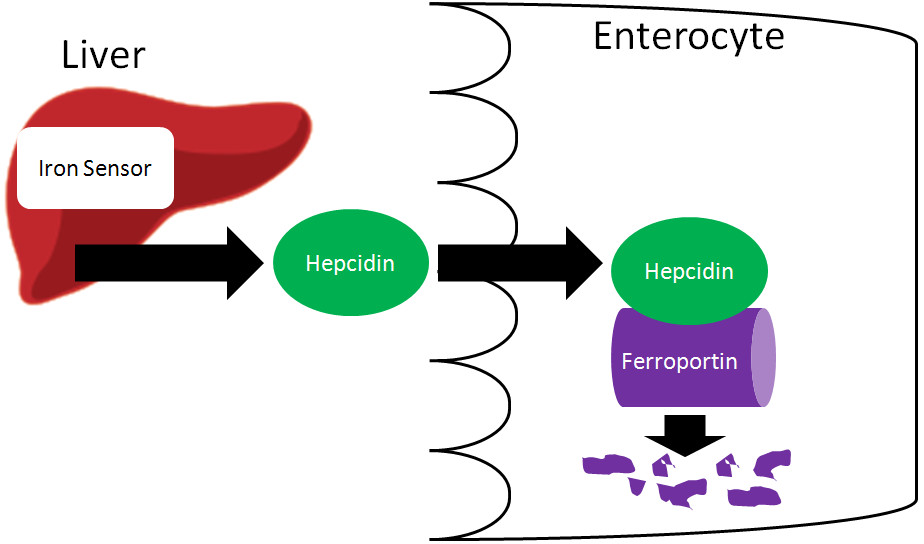 Illustration showing the action of hepcidin on ferroportin, preventing iron transport into circulation
Illustration showing the action of hepcidin on ferroportin, preventing iron transport into circulation
6. What Happens to Iron in Enterocytes If It Is Not Absorbed?
If iron is not absorbed, it remains trapped in enterocytes, which are eventually sloughed off and excreted in feces. This process helps regulate iron absorption by preventing excess iron from entering the bloodstream.
6.1. The Fate of Iron in Enterocytes
Enterocytes are the cells lining the small intestine responsible for absorbing nutrients, including iron, from the diet. Iron enters enterocytes via the divalent metal transporter 1 (DMT1). Once inside the enterocyte, iron can either be:
- Transported into the Bloodstream: Iron can be transported out of the enterocyte into the bloodstream via ferroportin, with the help of hephaestin to oxidize Fe2+ to Fe3+ for binding to transferrin.
- Stored as Ferritin: Iron can be stored as ferritin within the enterocyte.
6.2. Regulation by Hepcidin
The fate of iron in enterocytes is largely determined by hepcidin levels. When hepcidin levels are high, ferroportin is internalized and degraded, preventing iron from being transported out of the enterocyte. The iron then remains trapped in the enterocyte as ferritin.
6.3. Excretion of Iron
Enterocytes have a limited lifespan and are continuously sloughed off from the tips of the intestinal villi. When enterocytes containing iron-bound ferritin are sloughed off, they are excreted in the feces. This is a major route of iron excretion from the body.
6.4. Impact on Iron Balance
This process plays a crucial role in regulating iron balance. By trapping iron in enterocytes and excreting it in feces, the body can prevent excessive iron absorption when iron stores are high or when dietary intake is excessive.
6.5. Clinical Implications
Understanding this mechanism is important for managing iron-related disorders. For example, in iron deficiency anemia, strategies to enhance iron absorption may include consuming iron-rich foods and avoiding factors that inhibit iron absorption, such as certain medications or dietary components.
7. What Are the Key Differences in Iron Metabolism Between Men and Women?
Key differences in iron metabolism between men and women primarily stem from menstrual blood loss and pregnancy in women, leading to lower iron stores compared to men. Hormonal differences also influence iron regulation.
7.1. Iron Requirements
Men and women have different iron requirements due to physiological differences. Men generally require about 8 mg of iron per day, while women of childbearing age require 18 mg per day. This higher requirement in women is primarily due to menstrual blood loss.
7.2. Menstrual Blood Loss
Menstrual blood loss is a significant factor affecting iron metabolism in women. Each menstrual cycle, women lose iron through blood, which can deplete iron stores if not adequately replaced through diet or supplementation. The amount of iron lost varies among women, but on average, it is about 1 mg of iron per day over the course of the month.
7.3. Pregnancy
Pregnancy significantly increases iron requirements in women. During pregnancy, iron is needed to support the growth and development of the fetus, as well as to increase the mother’s blood volume. The recommended iron intake during pregnancy is 27 mg per day.
7.4. Iron Stores
Due to menstrual blood loss and pregnancy, women tend to have lower iron stores compared to men. This makes women more susceptible to iron deficiency anemia. Serum ferritin levels, which are an indicator of iron stores, are typically lower in women than in men.
7.5. Hormonal Influences
Hormones also play a role in iron metabolism. Estrogen, the primary female sex hormone, can influence hepcidin production and iron absorption. However, the exact mechanisms are still being studied.
7.6. Dietary Considerations
Given the differences in iron requirements, men and women should pay attention to their dietary iron intake. Iron-rich foods include red meat, poultry, fish, beans, and fortified cereals. Women, in particular, may benefit from consuming iron-rich foods and, in some cases, taking iron supplements to maintain adequate iron levels.
7.7. Summary of Differences
To summarize, here’s a table highlighting the key differences in iron metabolism between men and women:
| Feature | Men | Women |
|---|---|---|
| Iron Requirement | 8 mg/day | 18 mg/day (childbearing age), 27 mg/day (pregnancy) |
| Menstrual Blood Loss | Not applicable | Significant factor affecting iron levels |
| Pregnancy | Not applicable | Increases iron requirements |
| Iron Stores | Generally higher | Generally lower |
| Hormonal Influences | Less significant | Estrogen may influence hepcidin production |
| Risk of Iron Deficiency | Lower | Higher |
8. How Do Iron-Containing Enzymes Utilize Iron?
Iron-containing enzymes use iron as a cofactor to facilitate various biochemical reactions, including oxidation-reduction reactions, oxygen transport, and DNA synthesis, playing essential roles in cellular metabolism.
8.1. Iron as a Cofactor
Iron is an essential cofactor for many enzymes, meaning that it is required for the enzyme to function properly. Iron’s ability to readily accept and donate electrons makes it particularly useful in oxidation-reduction (redox) reactions.
8.2. Types of Iron-Containing Enzymes
There are several types of iron-containing enzymes, each with unique functions:
- Heme Enzymes: These enzymes contain heme, a porphyrin ring with a central iron atom. Examples include:
- Cytochromes: Involved in the electron transport chain in mitochondria, which is essential for energy production.
- Catalase: Protects cells from oxidative damage by converting hydrogen peroxide to water and oxygen.
- Peroxidases: Involved in various metabolic processes, including the detoxification of harmful substances.
- Iron-Sulfur Cluster Enzymes: These enzymes contain iron-sulfur clusters, which are groups of iron and sulfur atoms. Examples include:
- Aconitase: Involved in the citric acid cycle (Krebs cycle), a key metabolic pathway for energy production.
- Nitrogenase: Found in nitrogen-fixing bacteria, nitrogenase is essential for converting atmospheric nitrogen to ammonia, a form of nitrogen that plants can use.
- Complex I (NADH dehydrogenase): A component of the electron transport chain in mitochondria.
- Non-Heme Iron Enzymes: These enzymes contain iron but do not have heme or iron-sulfur clusters. Examples include:
- Ribonucleotide Reductase: Essential for DNA synthesis, converting ribonucleotides to deoxyribonucleotides.
- Phenylalanine Hydroxylase: Involved in the metabolism of phenylalanine, an amino acid.
8.3. Specific Examples of Iron-Containing Enzymes and Their Functions
To illustrate how iron-containing enzymes utilize iron, here are some specific examples:
- Cytochromes: These enzymes are crucial for the electron transport chain in mitochondria. Iron in the heme group of cytochromes accepts and donates electrons, facilitating the transfer of electrons from one molecule to another, ultimately leading to the production of ATP (energy).
- Catalase: This enzyme protects cells from oxidative damage by catalyzing the decomposition of hydrogen peroxide (H2O2) into water (H2O) and oxygen (O2). Iron in the heme group of catalase facilitates this reaction, preventing the accumulation of harmful free radicals.
- Ribonucleotide Reductase: This enzyme is essential for DNA synthesis, converting ribonucleotides to deoxyribonucleotides. Iron in the active site of ribonucleotide reductase facilitates this conversion, ensuring that cells have the building blocks needed for DNA replication.
8.4. Importance of Iron for Enzyme Function
Iron is essential for the proper function of these enzymes. Iron deficiency can impair the activity of iron-containing enzymes, leading to various health problems. For example, iron deficiency can impair the function of cytochromes, leading to reduced energy production and fatigue. It can also impair the function of ribonucleotide reductase, leading to impaired DNA synthesis and cell growth.
8.5. Summary of Iron Utilization in Enzymes
To summarize, iron is utilized in enzymes as follows:
| Enzyme Type | Function | Role of Iron |
|---|---|---|
| Heme Enzymes | Electron transport, antioxidant defense, metabolic processes | Accepts and donates electrons, facilitates redox reactions |
| Iron-Sulfur Enzymes | Energy production, nitrogen fixation, electron transport | Forms clusters that facilitate electron transfer, stabilizes enzyme structure |
| Non-Heme Iron Enzymes | DNA synthesis, amino acid metabolism | Facilitates conversion of molecules, essential for enzyme activity |
9. What Role Does the Liver Play in Iron Storage and Regulation?
The liver is a central organ in iron storage and regulation, storing iron as ferritin and hemosiderin, and producing hepcidin to control iron absorption and distribution throughout the body. Its functions are vital for maintaining iron homeostasis.
9.1. Iron Storage in the Liver
The liver is the primary site for iron storage in the body. Hepatocytes (liver cells) store iron in the form of ferritin and hemosiderin. These storage proteins help maintain iron in a non-toxic form and make it available when needed for various physiological processes.
9.2. Ferritin and Hemosiderin in the Liver
- Ferritin: The liver contains a high concentration of ferritin, the primary iron storage protein. Ferritin in the liver can store large amounts of iron, providing a readily available source of iron when needed.
- Hemosiderin: The liver also stores iron as hemosiderin, a long-term storage form of iron. Hemosiderin accumulates when iron levels are high, providing a means of storing excess iron.
9.3. Hepcidin Production
The liver produces hepcidin, a hormone that plays a central role in regulating iron homeostasis. Hepcidin controls the release of iron from enterocytes (intestinal cells) and macrophages (immune cells that recycle iron from red blood cells) into the bloodstream.
9.4. Regulation of Hepcidin Production
Hepcidin production is regulated by several factors, including:
- Iron Levels: High iron levels increase hepcidin production, while low iron levels decrease hepcidin production. This ensures that iron absorption is reduced when iron stores are high and increased when iron stores are low.
- Inflammation: Inflammatory cytokines, such as interleukin-6 (IL-6), increase hepcidin production. This is why chronic inflammation can lead to anemia of chronic disease, where iron is trapped in cells and unavailable for red blood cell production.
- Erythropoietic Activity: Increased erythropoietic activity (red blood cell production) decreases hepcidin production. This ensures that more iron is available for red blood cell synthesis when the body needs more red blood cells.
9.5. Clinical Significance
The liver’s role in iron storage and regulation is clinically significant. Liver diseases, such as hemochromatosis (iron overload disorder) and chronic liver inflammation, can disrupt iron metabolism and lead to iron-related disorders.
9.6. Liver Function Tests
Liver function tests, such as serum ferritin levels, can provide valuable information about iron status. Elevated serum ferritin levels can indicate iron overload or liver inflammation, while low levels can indicate iron deficiency.
9.7. Summary of the Liver’s Role
To summarize, the liver plays a critical role in iron storage and regulation as follows:
| Function | Description |
|---|---|
| Iron Storage | Stores iron as ferritin and hemosiderin, providing a readily available source of iron when needed |
| Hepcidin Production | Produces hepcidin, a hormone that regulates iron absorption and distribution |
| Regulation of Hepcidin | Regulates hepcidin production based on iron levels, inflammation, and erythropoietic activity |
| Clinical Significance | Liver diseases can disrupt iron metabolism and lead to iron-related disorders |
10. How Does Inflammation Affect Iron Transport and Storage in the Body?
Inflammation significantly affects iron transport and storage by increasing hepcidin production, which traps iron within cells and reduces its availability for red blood cell production, leading to anemia of chronic disease.
10.1. The Role of Hepcidin in Inflammation
Inflammation has a significant impact on iron metabolism through the action of hepcidin. Inflammatory cytokines, such as interleukin-6 (IL-6), stimulate the production of hepcidin by the liver.
10.2. Mechanism of Action
As mentioned earlier, hepcidin acts by binding to ferroportin, the only known iron exporter protein. When hepcidin binds to ferroportin, it causes ferroportin to be internalized and degraded. This prevents iron from being transported out of the cell and into the bloodstream.
10.3. Impact on Iron Transport
Inflammation-induced hepcidin production reduces iron transport from:
- Enterocytes: Reduced iron absorption from the diet.
- Macrophages: Reduced recycling of iron from old red blood cells.
10.4. Anemia of Chronic Disease
The combined effect of reduced iron absorption and reduced iron recycling leads to a condition known as anemia of chronic disease (also called anemia of inflammation). In this condition, iron is trapped in cells (enterocytes and macrophages) and is not available for red blood cell production.
10.5. Diagnostic Considerations
In anemia of chronic disease, serum iron levels are typically low, but serum ferritin levels are normal or elevated, reflecting the fact that iron is stored in cells but not available for use. This is in contrast to iron deficiency anemia, where both serum iron and serum ferritin levels are low.
10.6. Management Strategies
Managing anemia of chronic disease can be challenging. Treatment typically focuses on addressing the underlying inflammatory condition. In some cases, erythropoiesis-stimulating agents (ESAs) may be used to stimulate red blood cell production, but these agents can have side effects and are not always effective.
10.7. Summary of Inflammation’s Impact
To summarize, inflammation affects iron transport and storage as follows:
| Impact | Description |
|---|---|
| Increased Hepcidin | Inflammatory cytokines stimulate hepcidin production by the liver |
| Reduced Iron Transport | Hepcidin reduces iron transport from enterocytes and macrophages |
| Anemia of Inflammation | Iron is trapped in cells, leading to reduced red blood cell production and anemia |
| Diagnostic Indicators | Low serum iron, normal or elevated serum ferritin |
By understanding these mechanisms, healthcare professionals can better diagnose and manage iron-related disorders associated with inflammation. For more insights and detailed information on iron transport and storage, visit worldtransport.net, where we provide comprehensive resources on the latest advancements in transportation and logistics, including the intricate pathways of nutrient transport within the human body.
Navigating the complexities of iron transport and storage requires staying informed with the latest research and best practices. At worldtransport.net, we are committed to providing you with reliable and up-to-date information. Explore our extensive library of articles, case studies, and expert analyses to deepen your understanding and optimize your strategies.
Ready to take the next step? Visit worldtransport.net today and discover how our resources can help you achieve excellence in iron management. For further inquiries, contact us at:
Address: 200 E Randolph St, Chicago, IL 60601, United States
Phone: +1 (312) 742-2000
Website: worldtransport.net
FAQ: Understanding Iron Transport and Storage
1. Why is iron important for the body?
Iron is essential because it is a key component of hemoglobin, which carries oxygen in red blood cells, and myoglobin, which stores oxygen in muscles. It is also needed for energy production and the function of many enzymes.
2. What is transferrin, and what does it do?
Transferrin is a protein that transports iron in the bloodstream. It binds to iron and delivers it to cells throughout the body that have transferrin receptors.
3. How does iron get into cells?
Iron gets into cells through a process called endocytosis, where the transferrin-iron complex binds to a transferrin receptor on the cell surface, and the cell internalizes the complex.
4. What are ferritin and hemosiderin?
Ferritin and hemosiderin are iron storage proteins. Ferritin is the primary storage protein and releases iron when needed, while hemosiderin stores iron in a less accessible form and accumulates during iron overload.
5. Where is iron stored in the body?
Iron is mainly stored in the liver, spleen, and bone marrow.
6. How does the body regulate iron absorption?
The body regulates iron absorption through hepcidin, a hormone that controls the release of iron from enterocytes (intestinal cells) and macrophages into the bloodstream.
7. What is hepcidin, and how does it work?
Hepcidin is a hormone produced by the liver that binds to ferroportin, the iron exporter protein, causing it to degrade. This prevents iron from being transported out of cells, reducing iron absorption and recycling.
8. What happens to iron if it is not absorbed?
If iron is not absorbed, it remains trapped in enterocytes, which are eventually sloughed off and excreted in feces.
9. How does inflammation affect iron levels?
Inflammation increases hepcidin production, which traps iron within cells and reduces its availability for red blood cell production, leading to anemia of chronic disease.
10. What are the differences in iron metabolism between men and women?
Women have higher iron requirements than men due to menstrual blood loss and pregnancy. As a result, women tend to have lower iron stores and are more susceptible to iron deficiency anemia.

