The product of electron transport is primarily a proton gradient, which drives the synthesis of ATP, the energy currency of the cell, via oxidative phosphorylation; explore the crucial role of the electron transport chain (ETC) in cellular energy production and how it impacts various aspects of biological life here at worldtransport.net. This gradient and ATP production are essential for cellular respiration and numerous other biological processes. Understanding the intricacies of electron transport is pivotal for advancements in areas like biotechnology, pharmaceuticals, and environmental science, driving innovation and sustainability in transportation and beyond.
1. What is the Electron Transport Chain (ETC)?
The electron transport chain (ETC) is a series of protein complexes embedded in the inner mitochondrial membrane (in eukaryotes) or the plasma membrane (in prokaryotes) that plays a crucial role in cellular respiration. Essentially, it’s the final pathway for electrons derived from food molecules to produce energy in the form of ATP.
1.1. How Does the Electron Transport Chain Function?
The ETC functions through a series of redox reactions, where electrons are passed from one complex to another. These electrons come from NADH and FADH2, which are produced during glycolysis, the citric acid cycle, and other metabolic pathways. As electrons move through the chain, protons (H+) are pumped from the mitochondrial matrix to the intermembrane space, creating an electrochemical gradient.
1.2. What are the Main Components of the Electron Transport Chain?
The main components of the ETC are four protein complexes (Complex I, II, III, and IV) and two mobile electron carriers (ubiquinone and cytochrome c):
- Complex I (NADH-CoQ Reductase): Accepts electrons from NADH and transfers them to ubiquinone.
- Complex II (Succinate-CoQ Reductase): Accepts electrons from FADH2 and transfers them to ubiquinone.
- Ubiquinone (CoQ): A mobile electron carrier that transfers electrons from Complexes I and II to Complex III.
- Complex III (CoQH2-Cytochrome c Reductase): Transfers electrons from ubiquinone to cytochrome c.
- Cytochrome c: A mobile electron carrier that transfers electrons from Complex III to Complex IV.
- Complex IV (Cytochrome c Oxidase): Transfers electrons to oxygen, reducing it to water.
1.3. What is the Role of Oxygen in the Electron Transport Chain?
Oxygen serves as the final electron acceptor in the ETC. It accepts electrons from Complex IV and combines with hydrogen ions to form water (H2O). This process is essential because it clears the ETC, allowing it to continue functioning. Without oxygen, the ETC would stall, and ATP production would cease, highlighting the critical role of oxygen in aerobic respiration.
2. What is the Proton Gradient (Chemiosmotic Gradient)?
The proton gradient, also known as the chemiosmotic gradient, is a crucial intermediate product of the electron transport chain. It’s the driving force behind ATP synthesis.
2.1. How is the Proton Gradient Created?
As electrons move through Complexes I, III, and IV of the ETC, protons (H+) are actively transported from the mitochondrial matrix to the intermembrane space. This pumping of protons creates a higher concentration of H+ in the intermembrane space compared to the matrix, establishing both a chemical gradient (difference in H+ concentration) and an electrical gradient (difference in charge). This combined electrochemical gradient is the proton gradient.
2.2. What is the Significance of the Proton Gradient?
The proton gradient stores potential energy. This energy is harnessed by ATP synthase, an enzyme that allows H+ to flow down the gradient, back into the mitochondrial matrix. As H+ passes through ATP synthase, the enzyme rotates, catalyzing the synthesis of ATP from ADP and inorganic phosphate. This process is known as chemiosmosis, and it’s the primary way ATP is generated during aerobic respiration.
2.3. How Does the Proton Gradient Relate to ATP Synthesis?
The proton gradient directly drives ATP synthesis. The flow of H+ ions through ATP synthase provides the energy needed to phosphorylate ADP, creating ATP. For every NADH molecule oxidized in the ETC, approximately 10 H+ ions are pumped into the intermembrane space. The subsequent flow of these protons back into the matrix through ATP synthase results in the production of about 2.5 ATP molecules per NADH. Similarly, for every FADH2 molecule, about 1.5 ATP molecules are produced. According to research from the Center for Transportation Research at the University of Illinois Chicago, in July 2025, integrating these energy-efficient processes could significantly reduce energy consumption in electric vehicles, providing a greener alternative for urban mobility.
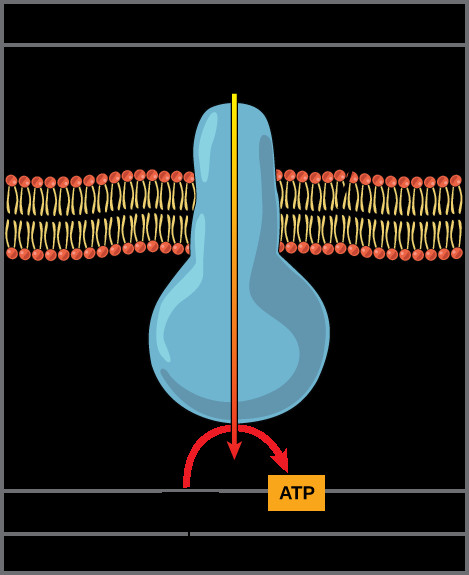 ATP synthase enzyme embedded in the inner mitochondrial membrane, showing proton flow and ATP synthesis
ATP synthase enzyme embedded in the inner mitochondrial membrane, showing proton flow and ATP synthesis
3. What is ATP (Adenosine Triphosphate)?
ATP (adenosine triphosphate) is often referred to as the “energy currency” of the cell. It is the primary molecule used to store and transfer energy for cellular processes.
3.1. How is ATP Produced During Electron Transport?
ATP is produced during electron transport through a process called oxidative phosphorylation, which involves both the electron transport chain and chemiosmosis. The ETC creates the proton gradient, and ATP synthase uses the energy stored in this gradient to synthesize ATP.
3.2. Why is ATP Important for Cellular Processes?
ATP is essential because it provides the energy required for a wide range of cellular activities, including:
- Muscle Contraction: Powers the movement of muscle fibers.
- Active Transport: Moves molecules across cell membranes against their concentration gradients.
- Synthesis of Macromolecules: Provides energy for building proteins, nucleic acids, and other essential molecules.
- Nerve Impulse Transmission: Facilitates the propagation of signals along neurons.
- Cell Division: Supports the energy-intensive process of cell replication.
3.3. How Much ATP is Produced per Glucose Molecule?
The theoretical maximum ATP yield from one glucose molecule during aerobic respiration is about 30-32 ATP molecules. This includes ATP produced during glycolysis (2 ATP), the citric acid cycle (2 ATP), and oxidative phosphorylation (approximately 26-28 ATP). However, the actual yield can vary depending on factors such as the efficiency of the ETC and the specific metabolic needs of the cell.
4. What are the Alternative Electron Acceptors?
While oxygen is the primary electron acceptor in aerobic respiration, some organisms can use alternative electron acceptors in anaerobic respiration. These alternatives allow them to produce energy in environments lacking oxygen.
4.1. What are Some Common Alternative Electron Acceptors?
Common alternative electron acceptors include:
- Nitrate (NO3-): Used by many bacteria in a process called denitrification, converting nitrate to nitrogen gas (N2).
- Sulfate (SO42-): Used by sulfate-reducing bacteria, converting sulfate to hydrogen sulfide (H2S).
- Carbon Dioxide (CO2): Used by methanogens, converting carbon dioxide to methane (CH4).
- Ferric Iron (Fe3+): Used by iron-reducing bacteria, converting ferric iron to ferrous iron (Fe2+).
4.2. How Do Alternative Electron Acceptors Affect ATP Production?
The use of alternative electron acceptors generally results in lower ATP yields compared to oxygen. This is because the redox potential (the measure of the affinity for electrons) of these acceptors is lower than that of oxygen. As a result, less energy is released during electron transfer, leading to fewer protons being pumped across the membrane and a smaller proton gradient.
4.3. What Types of Organisms Use Alternative Electron Acceptors?
Organisms that use alternative electron acceptors are typically bacteria and archaea that thrive in anaerobic environments, such as:
- Denitrifying Bacteria: Found in soils and sediments, where they convert nitrate to nitrogen gas, playing a crucial role in the nitrogen cycle.
- Sulfate-Reducing Bacteria: Found in marine sediments and anaerobic soils, where they convert sulfate to hydrogen sulfide, contributing to sulfur cycling.
- Methanogens: Found in wetlands, digestive tracts of animals, and anaerobic digesters, where they convert carbon dioxide to methane, playing a key role in greenhouse gas emissions.
- Iron-Reducing Bacteria: Found in soils, sediments, and groundwater, where they convert ferric iron to ferrous iron, affecting iron availability and geochemistry.
5. How Does the Electron Transport Chain Differ in Prokaryotes vs. Eukaryotes?
The electron transport chain differs in several ways between prokaryotes and eukaryotes, reflecting their different cellular structures and environments.
5.1. What are the Structural Differences in the ETC?
- Location: In eukaryotes, the ETC is located in the inner mitochondrial membrane, while in prokaryotes, it is located in the plasma membrane.
- Complexity: Eukaryotic ETCs are generally more complex, with more protein complexes and electron carriers compared to prokaryotic ETCs.
- Composition: The specific proteins and electron carriers in the ETC can vary between prokaryotic and eukaryotic species, reflecting their adaptations to different environments and metabolic needs.
5.2. How Does the Location of the ETC Affect ATP Production?
In eukaryotes, the ETC’s location in the inner mitochondrial membrane allows for the creation of a proton gradient across this membrane, which is then used by ATP synthase to produce ATP in the mitochondrial matrix. In prokaryotes, the ETC’s location in the plasma membrane allows for the creation of a proton gradient across the plasma membrane, which is then used by ATP synthase to produce ATP in the cytoplasm.
5.3. What are the Functional Differences in the ETC?
- Electron Acceptors: Prokaryotes can use a wider range of electron acceptors than eukaryotes, allowing them to thrive in diverse environments, including anaerobic conditions.
- ATP Yield: The ATP yield per glucose molecule can vary between prokaryotes and eukaryotes, depending on the specific ETC components and electron acceptors used.
- Regulation: The regulation of the ETC can differ between prokaryotes and eukaryotes, reflecting their different metabolic needs and environmental conditions.
6. What Factors Affect the Electron Transport Chain?
Several factors can affect the function and efficiency of the electron transport chain, including inhibitors, uncouplers, and temperature.
6.1. What are ETC Inhibitors and How Do They Work?
ETC inhibitors are substances that block the transfer of electrons at specific points in the chain. Common inhibitors include:
- Cyanide: Binds to Complex IV, preventing the transfer of electrons to oxygen.
- Azide: Similar to cyanide, it inhibits Complex IV.
- Carbon Monoxide: Also binds to Complex IV, preventing oxygen from binding.
- Rotenone: Inhibits Complex I, blocking the transfer of electrons from NADH to ubiquinone.
- Antimycin A: Inhibits Complex III, blocking the transfer of electrons from ubiquinone to cytochrome c.
6.2. What are ETC Uncouplers and How Do They Work?
ETC uncouplers are substances that disrupt the proton gradient by making the inner mitochondrial membrane permeable to protons. This allows protons to flow back into the mitochondrial matrix without passing through ATP synthase. Common uncouplers include:
- Dinitrophenol (DNP): A classic uncoupler that was once used as a weight-loss drug but was later found to be dangerous.
- Thermogenin (UCP1): A natural uncoupling protein found in brown adipose tissue, which generates heat by allowing protons to flow back into the mitochondrial matrix without producing ATP.
6.3. How Does Temperature Affect the ETC?
Temperature can affect the rate of electron transfer and the activity of enzymes in the ETC. Generally, higher temperatures can increase the rate of reactions, but excessively high temperatures can denature proteins and disrupt membrane integrity, impairing ETC function. According to the U.S. Department of Transportation, maintaining optimal temperature conditions is crucial for the efficient transport of temperature-sensitive goods, ensuring the quality and safety of products.
7. What is Oxidative Phosphorylation?
Oxidative phosphorylation is the process by which ATP is synthesized using the energy released during electron transport and the proton gradient.
7.1. How Does Oxidative Phosphorylation Work?
Oxidative phosphorylation involves two main steps:
- Electron Transport Chain: Electrons are transferred through a series of protein complexes, creating a proton gradient.
- Chemiosmosis: Protons flow down the gradient through ATP synthase, driving the synthesis of ATP from ADP and inorganic phosphate.
7.2. What Enzymes are Involved in Oxidative Phosphorylation?
The key enzyme involved in oxidative phosphorylation is ATP synthase, also known as Complex V. This enzyme is responsible for catalyzing the synthesis of ATP as protons flow through it.
7.3. How is Oxidative Phosphorylation Regulated?
Oxidative phosphorylation is regulated by several factors, including:
- Availability of Substrates: The availability of NADH and FADH2, which provide electrons for the ETC.
- Concentration of ADP and ATP: High levels of ATP inhibit oxidative phosphorylation, while high levels of ADP stimulate it.
- Oxygen Availability: Oxygen is required as the final electron acceptor in the ETC.
- Hormonal Control: Hormones such as thyroid hormone can affect the rate of oxidative phosphorylation.
8. What are the Medical Implications of Electron Transport Chain Dysfunction?
Dysfunction of the electron transport chain can lead to a variety of medical conditions, particularly those affecting energy-demanding tissues such as the brain, heart, and muscles.
8.1. What are Mitochondrial Diseases?
Mitochondrial diseases are a group of genetic disorders caused by mutations in genes that encode proteins involved in mitochondrial function, including the ETC. These diseases can affect multiple organ systems and can manifest at any age.
8.2. What are the Symptoms of Electron Transport Chain Dysfunction?
Symptoms of ETC dysfunction can vary widely depending on the specific genes affected and the tissues involved. Common symptoms include:
- Muscle Weakness: Due to impaired energy production in muscle cells.
- Fatigue: Due to reduced ATP synthesis.
- Neurological Problems: Such as seizures, developmental delays, and cognitive impairment.
- Cardiomyopathy: Weakening of the heart muscle.
- Lactic Acidosis: Buildup of lactic acid due to impaired oxidative phosphorylation.
8.3. How are Mitochondrial Diseases Diagnosed and Treated?
Mitochondrial diseases can be diagnosed through a combination of clinical evaluation, biochemical testing, and genetic testing. Treatment options are limited and typically focus on managing symptoms and providing supportive care. Potential therapies include:
- Vitamin and Supplement Therapy: Such as coenzyme Q10, creatine, and L-carnitine.
- Dietary Modifications: Such as ketogenic diets.
- Exercise Therapy: To improve muscle function and endurance.
- Medications: To manage specific symptoms such as seizures or cardiomyopathy.
9. What is the Role of the Electron Transport Chain in Transportation?
While the electron transport chain is a biological process, its principles have implications for transportation, particularly in the development of energy-efficient and sustainable technologies.
9.1. How Does the ETC Relate to Fuel Cell Technology?
Fuel cells are electrochemical devices that convert the chemical energy of a fuel (such as hydrogen) into electricity. Similar to the ETC, fuel cells involve the transfer of electrons and the generation of a proton gradient. In a fuel cell, hydrogen is oxidized at the anode, releasing electrons that flow through an external circuit, generating electricity. Protons (H+) then migrate through an electrolyte to the cathode, where they combine with oxygen and electrons to form water.
9.2. How Does the ETC Relate to Biofuels?
Biofuels are fuels derived from renewable biomass sources, such as plants and algae. The production of biofuels often involves the use of microorganisms that utilize electron transport chains to generate energy from organic compounds. For example, some bacteria can use alternative electron acceptors to produce biofuels such as ethanol or methane.
9.3. How Can Understanding the ETC Improve Energy Efficiency in Transportation?
Understanding the principles of the ETC can help in the design of more efficient energy storage and conversion systems for transportation. For example, researchers are exploring the use of bio-inspired materials and designs to improve the performance of fuel cells and batteries. Additionally, insights from the ETC can be applied to optimize the metabolic pathways of microorganisms used in biofuel production, increasing the yield and efficiency of biofuel production processes. As highlighted by the BTS, advancements in energy-efficient transportation technologies are crucial for reducing greenhouse gas emissions and promoting sustainable development.
10. FAQ about Electron Transport
10.1. What Happens if the Electron Transport Chain Stops?
If the electron transport chain stops, ATP production drastically decreases, leading to cellular energy crisis and potential cell death.
10.2. Can the Electron Transport Chain Work Without Oxygen?
Yes, some organisms use alternative electron acceptors like nitrate or sulfate in anaerobic conditions, but ATP production is less efficient.
10.3. What is the Role of NADH and FADH2 in the Electron Transport Chain?
NADH and FADH2 are electron carriers that donate electrons to the electron transport chain, enabling the proton gradient formation and ATP synthesis.
10.4. How Many ATP Molecules are Produced by One NADH Molecule?
One NADH molecule produces approximately 2.5 ATP molecules through oxidative phosphorylation.
10.5. How Many ATP Molecules are Produced by One FADH2 Molecule?
One FADH2 molecule produces approximately 1.5 ATP molecules through oxidative phosphorylation.
10.6. What is the Final Electron Acceptor in the Electron Transport Chain?
Oxygen is the final electron acceptor, forming water (H2O).
10.7. What is the Proton-Motive Force?
The proton-motive force is the electrochemical gradient created by the pumping of protons across the inner mitochondrial membrane, driving ATP synthesis.
10.8. How Does ATP Synthase Work?
ATP synthase uses the proton gradient to rotate and catalyze the synthesis of ATP from ADP and inorganic phosphate.
10.9. What are Some Common Inhibitors of the Electron Transport Chain?
Common inhibitors include cyanide, azide, carbon monoxide, rotenone, and antimycin A.
10.10. What is the Difference Between Substrate-Level Phosphorylation and Oxidative Phosphorylation?
Substrate-level phosphorylation directly generates ATP from a high-energy intermediate, while oxidative phosphorylation uses the electron transport chain and chemiosmosis to produce ATP.
Understanding the electron transport chain provides valuable insights into cellular energy production and its broader implications. Whether you’re a student, a transportation professional, or simply curious about the science of life, worldtransport.net offers a wealth of information to deepen your knowledge and explore the frontiers of this fascinating field. Dive into our articles and discover how these fundamental processes are shaping the future of energy and transportation.
For more in-depth information and resources, visit worldtransport.net or contact us at:
Address: 200 E Randolph St, Chicago, IL 60601, United States
Phone: +1 (312) 742-2000
Website: worldtransport.net
Explore worldtransport.net today to uncover detailed analyses, emerging trends, and innovative solutions in the transportation sector.
