Cellular respiration is a fundamental process of life, converting nutrients into usable energy in the form of ATP. While glycolysis and the citric acid cycle lay the groundwork, the majority of ATP production occurs through the electron transport chain, often visualized and understood using an Electron Transport Diagram. This intricate system, embedded within the mitochondrial membrane, harnesses the power of redox reactions to generate a proton gradient, which ultimately drives ATP synthesis. Let’s delve into the complexities of this vital process and understand how an electron transport diagram helps illuminate its mechanisms.
The electron transport chain (ETC) is the final stage of aerobic respiration and crucially depends on oxygen as the terminal electron acceptor. Imagine it as a biological assembly line, where electrons are passed down a series of protein complexes, much like a relay race. This transfer of electrons, through a series of oxidation-reduction (redox) reactions, releases energy that is used to pump protons across the inner mitochondrial membrane. This pumping action creates an electrochemical gradient, a reservoir of potential energy that will be tapped to produce ATP.
The ETC is composed of four major protein complexes, labeled I to IV in diagrams, along with mobile electron carriers. These components are strategically located in the inner mitochondrial membrane in eukaryotes and the plasma membrane in prokaryotes. While oxygen is typically the final electron acceptor, it’s worth noting that some prokaryotes in anaerobic environments utilize alternative acceptors. However, the core principle of an electron transport chain remains consistent: to establish a proton gradient across a membrane.
Complex I: NADH Dehydrogenase
The journey begins at Complex I, also known as NADH dehydrogenase. NADH, generated from earlier stages of cellular respiration like the citric acid cycle, delivers two high-energy electrons to this complex. Complex I is a massive protein, featuring flavin mononucleotide (FMN) and iron-sulfur (Fe-S) proteins. FMN, a derivative of vitamin B2 (riboflavin), acts as a crucial prosthetic group – a non-protein component essential for protein function. In fact, the ETC is rich in prosthetic groups, including coenzymes, which are vital for enzyme activity within the complexes.
Complex I functions as a proton pump, utilizing the energy released from electron transfer to move four hydrogen ions (protons) from the mitochondrial matrix to the intermembrane space. This action initiates the crucial proton gradient, the driving force for ATP synthesis.
Ubiquinone (Q) and Complex II: Succinate Dehydrogenase
Complex II presents a slightly different entry point into the ETC. It directly receives electrons from FADH2, another electron carrier produced in the citric acid cycle, bypassing Complex I. The linchpin connecting Complexes I and II to Complex III is ubiquinone (Q), a mobile, lipid-soluble molecule. Q navigates freely within the inner mitochondrial membrane’s hydrophobic core.
Upon reduction, ubiquinone (QH2) carries electrons from both Complex I (via NADH) and Complex II (via FADH2) to Complex III. Complex II includes succinate dehydrogenase, an enzyme from the citric acid cycle. Electrons entering through Complex II, originating from FADH2, contribute to ATP production but yield fewer ATP molecules compared to NADH. This is because they bypass the proton pump in Complex I, resulting in fewer protons being pumped across the membrane at this initial stage. The number of ATP molecules produced is directly related to the magnitude of the proton gradient.
Complex III: Cytochrome bc1 Complex
Complex III, also known as cytochrome bc1 complex or cytochrome oxidoreductase, is the next station in the electron transport chain. This complex comprises cytochrome b, iron-sulfur proteins (Rieske center), and cytochrome c proteins. Cytochromes are characterized by heme prosthetic groups, similar to heme in hemoglobin but specialized for electron transport, not oxygen binding. The iron ion at the heart of heme undergoes redox reactions, cycling between Fe++ (reduced) and Fe+++ (oxidized) states as electrons pass through.
Complex III continues the proton pumping, moving protons across the membrane, further enhancing the proton gradient. It then transfers electrons to cytochrome c, another mobile electron carrier, for delivery to Complex IV. Cytochrome c, unlike ubiquinone which carries electron pairs, accepts and carries single electrons.
Complex IV: Cytochrome c Oxidase
The final protein complex in the ETC is Complex IV, or cytochrome c oxidase. This complex is more intricate, containing cytochromes c, a, and a3, along with copper ions. Cytochrome c oxidase is the critical point where molecular oxygen is reduced. It holds an oxygen molecule tightly between iron and copper ions until it’s fully reduced. The reduced oxygen then rapidly picks up hydrogen ions from the mitochondrial matrix to form water (H2O).
 Electron transport chain embedded in the inner mitochondrial membrane.
Electron transport chain embedded in the inner mitochondrial membrane.
The consumption of hydrogen ions in water formation within the matrix also contributes to the proton gradient across the inner mitochondrial membrane. By removing protons from the matrix side, Complex IV reinforces the electrochemical gradient established by the preceding complexes.
Chemiosmosis and ATP Synthase
The electron transport chain, through the action of Complexes I, III, and IV, creates an electrochemical gradient, also known as a proton-motive force. This gradient represents stored energy, with a higher concentration of protons in the intermembrane space compared to the mitochondrial matrix.
Chemiosmosis is the process where this stored energy is harnessed to generate ATP. Protons, driven by their concentration and electrical gradients, flow back across the inner mitochondrial membrane into the matrix. However, they can only do so through a specific channel: ATP synthase.
ATP synthase is a remarkable molecular machine embedded in the inner mitochondrial membrane. It acts as a channel for proton flow and, simultaneously, as an enzyme that synthesizes ATP. The flow of protons through ATP synthase provides the energy to drive the phosphorylation of ADP (adenosine diphosphate) with inorganic phosphate (Pi), producing ATP (adenosine triphosphate). This process, linking the proton gradient to ATP synthesis, is termed oxidative phosphorylation.
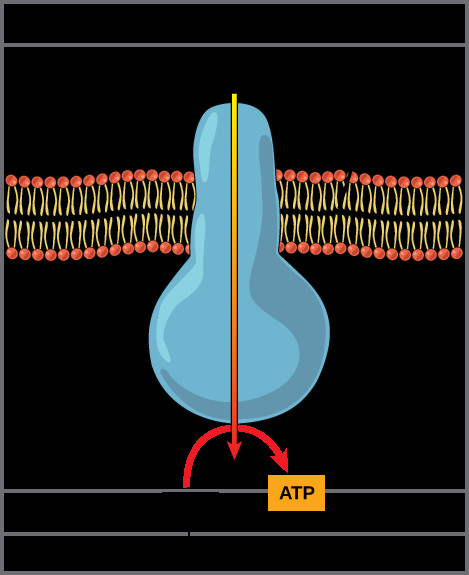 ATP synthase enzyme embedded in the inner mitochondrial membrane.
ATP synthase enzyme embedded in the inner mitochondrial membrane.
ATP Yield and Efficiency
The ATP yield from glucose catabolism is not fixed but varies depending on several factors. The efficiency of proton pumping by ETC complexes can differ slightly across species. Furthermore, the shuttle mechanisms transporting electrons from cytosolic NADH (generated during glycolysis) into the mitochondria can influence ATP yield. Depending on the shuttle used, electrons may enter the ETC via NAD+ or FAD+, with FAD+ leading to slightly less ATP production.
Cellular metabolism is also interconnected. Intermediates from glucose catabolism can be diverted into other biosynthetic pathways, and conversely, other biomolecules can feed into these energy-generating pathways. Despite these variations, the electron transport chain and chemiosmosis are remarkably efficient, capturing a significant portion of the energy from glucose. In living systems, glucose catabolism, including the electron transport chain, extracts approximately 34 percent of the energy stored in a glucose molecule.
Electron Transport Diagram: A Visual Summary
In essence, an electron transport diagram visually represents the flow of electrons through the ETC complexes, highlighting the redox reactions, proton pumping, and the ultimate generation of ATP via chemiosmosis. It’s a powerful tool for understanding this complex process.
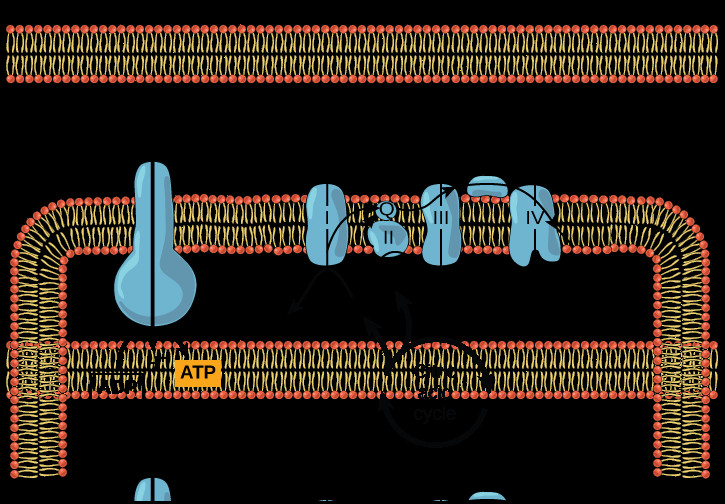 Electron transport chain, ATP synthase, and citric acid cycle in mitochondria.
Electron transport chain, ATP synthase, and citric acid cycle in mitochondria.
The electron transport chain is the cornerstone of aerobic respiration. By understanding the components and processes depicted in an electron transport diagram, we gain crucial insights into how cells efficiently convert energy from nutrients into the life-sustaining molecule, ATP. This intricate system, driven by redox reactions and chemiosmosis, underscores the elegance and efficiency of biological energy conversion.
