Introduction
Heart failure stands as a significant global health crisis, affecting an estimated 64.34 million individuals worldwide and contributing to 9.91 million disability-adjusted life years lost.1 The prevalence of this condition escalates with age and frequently coexists with type 2 diabetes mellitus, hypertension, and obesity. Notably, individuals with type 2 diabetes face a 2.5 times higher risk of developing heart failure compared to their non-diabetic counterparts.2 While concerns surrounding cardiovascular complications in type 2 diabetes have historically centered on atherosclerotic events, advancements in treating myocardial infarction, stroke, and limb ischemia have shifted the focus, recognizing heart failure as an early and critical complication.3
The challenge of managing heart failure in diabetic patients is further complicated by the limitations and potential risks associated with certain glucose-lowering agents. Thiazolidinediones, like pioglitazone, enhance insulin sensitivity but elevate the risk of heart failure by 42% due to sodium and fluid retention.4 Dipeptidyl peptidase 4 inhibitors present a mixed profile; while generally neutral on major cardiovascular events, saxagliptin, a member of this class, has been linked to increased heart failure hospitalization (27%) and cardiovascular mortality (22%).5 Insulin therapy, despite its glucose-lowering efficacy, is also concerning. A meta-analysis and real-world data analysis revealed a 27% and 2.02 odds ratio increase in all-cause mortality, and a 23% and 1.42 odds ratio increase in heart failure hospitalization, respectively, associated with insulin use in type 2 diabetes.6 These findings underscore the pressing need for safer and more effective glucose-lowering treatments for individuals with type 2 diabetes and heart failure.
The Rise of Sodium-Glucose Co-Transporter 2 (SGLT2) Inhibitors
Sodium-glucose co-transporter 2 (SGLT2) inhibitors represent a novel class of glucose-lowering medications. Their mechanism of action involves selectively blocking the SGLT2 protein, predominantly found in the proximal convoluted tubule of the nephron. This protein is responsible for reabsorbing approximately 90% of filtered glucose, with SGLT1 proteins handling the remaining glucose reabsorption in the distal proximal convoluted tubule. By inhibiting SGLT2, these drugs induce glycosuria (glucose excretion in urine) and natriuresis (sodium excretion), effectively lowering plasma glucose levels.7 8 This mechanism is distinct from other glucose-lowering agents as it operates independently of endogenous insulin or incretin pathways.
Cardiovascular outcome trials have demonstrated that SGLT2 inhibitors are associated with a significant 30%–35% reduction in the risk of hospitalization for heart failure.9–12 While other glucose-lowering drugs may exhibit greater potency in reducing glucose levels, they often fail to provide cardiovascular benefits, especially concerning heart failure outcomes. Intriguingly, the cardiovascular benefits of SGLT2 inhibitors persist even with declining kidney function, despite reduced glucose-lowering efficacy at lower estimated glomerular filtration rates. This suggests that the mechanisms driving glycemic control and cardiovascular risk reduction are distinct.13 This review aims to explore the key theories behind these cardioprotective mechanisms (table 1), which could pave the way for developing innovative therapies for both heart failure and type 2 diabetes.
View this table:
Table 1Potential mechanisms of cardiovascular benefits associated with SGLT2-inhibitor therapy
SGLT2 Inhibitors and Cardiovascular Outcomes: Clinical Trial Evidence
Driven by concerns about cardiovascular risks associated with some diabetes treatments, regulatory bodies in the USA and Europe mandated cardiovascular safety trials for all new hypoglycemic drugs in 2008. These trials have revealed that glucagon-like peptide 1 receptor agonists (GLP-1 RAs) and SGLT2 inhibitors can reduce cardiovascular events, reversing the trend seen with earlier therapies. While GLP-1 RAs primarily reduce atherothrombotic events such as myocardial infarction and stroke,14 SGLT2 inhibitors have consistently shown improved outcomes in heart failure9 10 15 (table 2). Notably, the reduction in heart failure hospitalization with SGLT2 inhibitors was observed within months of starting treatment, suggesting a rapid mechanism of action unlike other glucose-lowering drugs which often take years to show cardiovascular benefits.9–12
The consistent positive signal in early trials led to the ‘Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction’ (DAPA-HF) trial. This landmark trial specifically investigated the efficacy of dapagliflozin in heart failure patients with reduced ejection fraction, irrespective of diabetes status. The results demonstrated a 26% reduction in the risk of worsening heart failure or cardiovascular death with dapagliflozin, highlighting that these benefits are independent of glucose-lowering effects.15 Further solidifying these findings, the ‘Empagliflozin outcome trial in patients with chronic heart failure with reduced ejection fraction’ (EMPEROR-Reduced) trial showed that empagliflozin also reduced the composite risk of cardiovascular death and heart failure hospitalization in a similar patient population, again regardless of diabetes status.16 In both DAPA-HF and EMPEROR-Reduced trials, the primary outcome benefits were largely driven by a reduction in hospitalizations for heart failure.15 16 A meta-analysis of these two trials confirmed the consistent efficacy of both dapagliflozin and empagliflozin, demonstrating reductions in all-cause death, cardiovascular death, heart failure hospitalization, and decline in renal function.17 These compelling results suggest that the significant benefits are a class effect of SGLT2 inhibitors. The crucial question then becomes: what are the underlying mechanisms driving these remarkable benefits?
View this table:
Table 2Summary of large clinical trials of the SGLT2-inhibitor class
Conventional Theories Behind SGLT2 Inhibitor Benefits
The beneficial cardiovascular effects of SGLT2 inhibitors are likely multifaceted, with ongoing research exploring key pathways. Several conventional theories are discussed below.
Diuretic and Antihypertensive Effects: Managing Fluid Overload
One proposed mechanism for the cardiovascular benefits of SGLT2 inhibitors is their diuretic and antihypertensive actions (figure 1). SGLT2 inhibitors induce osmotic diuresis due to glycosuria and natriuresis. However, the precise nature of this diuresis (ratio of natriuresis to aquaresis) and its clinical significance are still under investigation.18 The contribution of osmotic diuresis to improved heart failure outcomes, particularly in patients already on loop diuretics, remains unclear. Glycosuria with SGLT2 inhibitors is glucose-dependent, occurring because of increased filtered and resorbed glucose in hyperglycemia. This glucose dependency raises questions about whether diuresis is the primary mechanism for benefits observed in normoglycemic heart failure patients in trials like DAPA-HF and EMPEROR-Reduced.15 16 While SGLT2 inhibitors do cause natriuresis and reduce plasma volume,9 19 it is uncertain if these effects are sustained and directly responsible for the heart failure benefits. Notably, NT-pro BNP levels, a marker of cardiac stress related to fluid overload, did not significantly change in chronic stable heart failure patients treated with SGLT2 inhibitors, despite improvements in heart failure status.20 Furthermore, diuretic doses remained largely unchanged and similar between SGLT2 inhibitor and placebo groups in the DAPA-HF trial.15
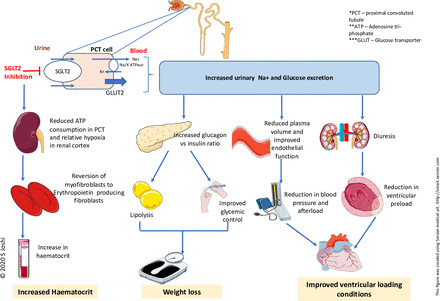 Figure 1
Figure 1
Figure 1Diagram illustrating traditional mechanisms of SGLT2 inhibitors: diuretic, antihypertensive, weight and glycemic control, and hematocrit increase.
Recent research highlights distinctions between SGLT2 inhibitor and loop diuretic effects. Mathematical modeling suggests dapagliflozin and bumetanide both reduce sodium and interstitial fluid. However, dapagliflozin has minimal impact on plasma volume, while bumetanide reduces intravascular volume, potentially leading to hypoperfusion issues.21 This interstitial fluid selectivity of SGLT2 inhibitors is supported by the RECEDE-CHF trial, showing that SGLT2 inhibitors increased daily urine volume by 545 mL, with 312 mL being electrolyte-free water clearance.22 This suggests a possible synergistic effect with background diuretics in heart failure, but more research is needed.
The antihypertensive effect of SGLT2 inhibitors, initially attributed to diuresis and natriuresis, is now considered to be potentially linked to improved endothelial function, reduced arterial stiffness, and altered sympathetic nervous activity, especially as it persists even with declining kidney function.23 24 However, a meta-analysis indicated only a modest blood pressure reduction (2.46/1.46 mm Hg) with SGLT2 inhibitors.25 While beneficial for cardiovascular health, this small blood pressure decrease is unlikely to fully explain the substantial improvements in cardiovascular morbidity and mortality seen with these drugs.
Weight Reduction and Glycemic Control: Contributing Factors?
Weight loss and improved glycemic control have also been proposed as mechanisms for the cardioprotective effects of SGLT2 inhibitors. SGLT2 inhibitors promote weight loss through an increased glucagon:insulin ratio, enhancing lipid mobilization, which may contribute to reduced heart failure mortality.26 27 Weight loss of up to 2.7 kg has been observed in type 2 diabetes patients on SGLT2 inhibitors, and some studies suggest weight loss in pre-diabetic individuals.28 29 However, current evidence does not support weight loss in heart failure patients without diabetes, questioning whether weight reduction is the primary mechanism. Furthermore, despite the prevalence of obesity in heart failure, definitive evidence on the impact of weight loss on cardiac function, quality of life, and exercise tolerance in these patients is limited.30 Therefore, weight loss alone is insufficient to explain the heart failure benefits of SGLT2 inhibitors.
Increased Hematocrit: A Potential but Unlikely Driver
SGLT2 inhibitors are known to increase renal erythropoietin production, red blood cell mass, and hematocrit.27 These changes could contribute to cardiovascular improvements. However, similar hematocrit increases with darbepoetin alfa did not show mortality benefits in patients with left ventricular systolic dysfunction.31 This suggests that while hematocrit increase might play a role, it’s unlikely to be the primary driver of the substantial heart failure benefits seen with SGLT2 inhibitors.
In summary, while diuretic, antihypertensive, weight reduction, and hematocrit increasing effects (figure 1) are associated with reduced cardiovascular risk, the modest nature of these effects with SGLT2 inhibitors does not fully account for the significant improvements observed in heart failure outcomes in clinical trials. Further investigation into more direct and novel mechanisms is warranted.
Unveiling Novel Mechanisms of SGLT2 Inhibitor Benefits
Direct Cardiac Effects: Beyond Hemodynamics
Cardiac hypertrophy, fibrosis, and inflammation are key drivers of adverse cardiac remodeling in heart failure, contributing to disease severity.32 Pre-clinical and clinical studies suggest that SGLT2 inhibitors may play a role in reversing this adverse remodeling.33 34 While this effect has been observed in type 2 diabetes patients with left ventricular hypertrophy, it has not been consistently demonstrated in heart failure patients.35 36 This discrepancy, especially given the prominent heart failure benefits of SGLT2 inhibitors, raises the possibility of novel, direct cardioprotective effects beyond ventricular unloading and remodeling (figure 2).
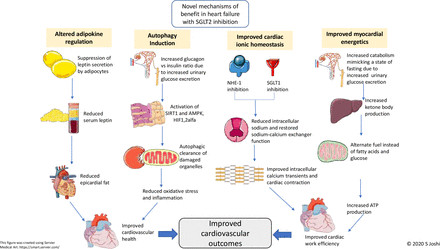 Figure 2
Figure 2
Figure 2Schematic representation of novel SGLT2 inhibitor mechanisms: improved myocardial energetics, ionic homeostasis, autophagy, and adipokine regulation.
Enhanced Myocardial Energetics: Fueling the Failing Heart
Under normal conditions, the heart derives approximately 90% of its energy from mitochondrial oxidative metabolism, primarily using free fatty acids, glucose, and to a lesser extent, lactate, ketones, and amino acids.37 In type 2 diabetes and heart failure, dysregulated fatty acid oxidation and impaired glucose uptake/oxidation lead to myocardial dysfunction. In this energy-compromised state, ketone bodies emerge as a “super-fuel,” producing ATP more efficiently than glucose or fatty acids. SGLT2 inhibitors promote hepatic ketone synthesis and reduce urinary ketone excretion, inducing a mild and persistent hyperketonemia. Beta-hydroxybutyrate, a key ketone body, is readily taken up by the heart and kidneys and preferentially oxidized over fatty acids and glucose. In animal models, beta-hydroxybutyrate improves cardiac work and reduces oxygen consumption, enhancing cardiac efficiency.38 This suggests that the cardiovascular benefits of SGLT2 inhibitors may be linked to a metabolic shift in the heart, moving away from fatty acid and glucose oxidation towards more oxygen-efficient ketone body metabolism, thereby improving cardiac energy utilization.
Restoring Myocardial Ionic Homeostasis: Calcium and Sodium Balance
Calcium homeostasis is critical for efficient heart muscle contraction (excitation-contraction coupling).39 During systole, calcium influx into cardiomyocytes via L-type calcium channels triggers calcium release from the sarcoplasmic reticulum (calcium-induced calcium release). This activates contractile proteins, leading to myocardial contraction. In type 2 diabetes and heart failure, sodium-hydrogen exchanger 1 and SGLT1 are upregulated, resulting in increased intracellular sodium levels.40 41 This excess sodium promotes calcium influx via sodium-calcium exchangers and calcium efflux from mitochondria, leading to elevated baseline intracellular calcium and reduced calcium transients and sarcoplasmic reticulum calcium stores in diabetic cardiomyocytes, impairing contractility.42
SGLT2 inhibitors reduce cardiac cytosolic sodium content by inhibiting sodium-hydrogen exchanger 1 and SGLT1 in diabetic rat and mouse myocytes, reversing calcium overload.42–47 Importantly, this effect on sodium-hydrogen exchanger 1 and SGLT1 is independent of diabetes status.48 These findings indicate that altered myocardial calcium handling plays a role in diabetic cardiomyopathy and heart failure, and SGLT2 inhibitors may improve the electrochemical environment in the failing heart by restoring ionic homeostasis, contributing to their cardiovascular benefits. A clinical trial is currently underway to further investigate the role of calcium handling in diabetic cardiomyopathy and heart failure and the effects of SGLT2 inhibitors on cardiac calcium homeostasis (NCT04591639). This study employs manganese-enhanced MRI, a novel imaging technique where manganese acts as a calcium analog, to assess myocardial calcium handling.
Autophagy: Cellular Housekeeping in the Heart
Autophagy is a cellular process essential for maintaining cellular health by removing damaged components and recycling cellular materials in response to stress, including hypoxia and nutrient deprivation.49 Experimental induction of autophagy has shown beneficial effects in heart failure by promoting the removal of dysfunctional mitochondria, a major source of damaging reactive oxygen species, which contribute to oxidative stress and inflammation.50 Autophagy pathways are activated by AMPK, SIRT1, and HIFs.51 It is hypothesized that SGLT2 inhibitors may induce autophagy by mimicking nutrient depletion through persistent glucosuria-induced catabolism. Indeed, various SGLT2 inhibitors have been shown to upregulate AMPK, SIRT1, and HIF-1 alfa.52 53 These autophagy-promoting actions may contribute to the observed cardiovascular benefits of SGLT2 inhibitors.54
Modulation of Adipokines: Leptin and Adiponectin Balance
Leptin and adiponectin, adipokines secreted by adipocytes, play crucial roles in regulating energy balance and food intake. Leptin is implicated in obesity-related cardiovascular diseases, while adiponectin is considered cardioprotective.55 Dysregulation of adiponectin and leptin, leading to epicardial fat deposition, is implicated in heart failure development.56 Elevated serum leptin levels in heart failure are associated with cardiac remodeling driven by fibrosis and inflammation.57
SGLT2 inhibitors have been shown to reduce serum leptin and increase adiponectin concentrations, potentially offering cardioprotection.58 However, these effects may be secondary to the systemic effects of SGLT2 inhibitors, such as weight loss and lipolysis, rather than a primary mechanism of direct cardiac benefit.
Future Research Directions
The precise mechanisms underlying the cardiovascular benefits of SGLT2 inhibitors are still being elucidated, and further novel mechanisms may emerge. It is plausible that SGLT2 inhibition interacts with or modulates other critical cellular pathways to exert its cardioprotective effects. Identifying these exact mechanisms is crucial for a comprehensive understanding of SGLT2 inhibitor benefits and could reveal new pathways for future heart failure therapies.
Ongoing clinical studies are actively investigating the mechanisms of cardiovascular benefits with SGLT2 inhibitors, focusing on cardiac remodeling (NCT03871621), lipolysis and epicardial fat modification (NCT04219124, NCT04167761 and NCT02235298), myocardial calcium handling (NCT04591639), and endogenous ketone production (NCT03852901, NCT04219124).
These ongoing studies are expected to provide valuable insights into the key mechanisms driving the cardioprotective effects of SGLT2 inhibitors.
Conclusion
Sodium-glucose co-transporter 2 (SGLT2) inhibitors represent a groundbreaking class of medications for managing type 2 diabetes and heart failure. Their cardioprotective effects have been consistently demonstrated across models of diabetic cardiomyopathy, heart failure, and ischemic cardiomyopathy. While the precise mechanism of benefit in heart failure remains under investigation, it likely involves a combination of systemic and direct myocardial effects. Strong pre-clinical evidence suggests that improved myocardial ionic homeostasis is a significant contributor to the cardiovascular benefits observed with SGLT2 inhibitors. Continued research into the mechanistic role of SGLT2 inhibitors in cardiovascular health is crucial for advancing our understanding of heart failure and diabetic cardiomyopathy, potentially paving the way for novel therapeutic strategies targeting these pathways.
Ethics statements
Patient consent for publication
Not required.
References
[1] GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789–858.
[2] Nichols GA, Hillier TA, Erbey JR, et al. Progression from newly diagnosed diabetes to incident heart failure. Diabetes Care 2001;24:714–9.
[3] 기동훈. 2020 Korean Diabetes Association Clinical Practice Guidelines for Diabetes. Diabetes Metab J 2021;45:1–33.
[4] Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes treated with rosiglitazone: a systematic review and meta-analysis. Diabetes Care 2007;30:2420–3.
[5] Udell JA, Cavender MA, Bhatt DL, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–26.
[6] Eurich DT, Simpson SH, Majumdar SR, et al. Comparative safety and effectiveness of метаformin, sulfonylureas and инсулин monotherapy for the treatment of type 2 diabetes: a retrospective cohort study. Diabetologia 2008;51:1307–14.
[7] DeFronzo RA, Norton L, Abdul-Ghani M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibitors. Nat Rev Nephrol 2017;13:11–26.
[8] Vallon V, Thomson SC. Renal function in the pathogenesis of the cardiorenal metabolic syndrome. Pflugers Arch 2017;469:513–23.
[9] Zinman B, Wanner LI, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–28.
[10] Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–57.
[11] Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–57.
[12] Kato ET, Silverman MG, Mosenzon O, et al. Effect of ertugliflozin on cardiovascular outcomes in type 2 diabetes mellitus participants with and without heart failure: results from VERTIS CV. Circulation 2019;140:1285–97.
[13] Cherney DZI, Perkins BA, Soleymanlou N, et al. Sodium-glucose cotransporter 2 inhibitor dapagliflozin in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2014;2:779–86.
[14] Sattar N, Lee MMY, Kristensen SL, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol 2021;9:819–28.
[15] McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008.
[16] Packer CJ, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–24.
[17] Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020;396:1911–20.
[18] Chilton R, Tikkanen I, Cannon CP, et al. Effects of sodium-glucose cotransporter 2 inhibitors on blood pressure, body weight, and urine albumin-to-creatinine ratio during routine care in patients with type 2 diabetes: data from the DECLARE-TIMI 58 trial. Circulation 2021;144:38–49.
[19] van Bommel RJ, Muskiet MHA, van Veldhuisen DJ, et al. The renal effects of sodium-glucose co-transporter 2 inhibitors in heart failure: a systematic review. Eur J Heart Fail 2017;19:1571–81.
[20] Løkke M, Galløe AM, Løgstrup BB, et al. Effects of empagliflozin on natriuretic peptides and cardiac biomarkers in patients with type 2 diabetes and chronic heart failure. JACC Heart Fail 2020;8:743–52.
[21] Hallow KM, Greasley PJ, Helbert L, et al. Sodium glucose cotransporter 2 inhibitor preferentially removes interstitial fluid in humans. JCI Insight 2018;3:e124559.
[22] Nijhoff MF, Laverman GD, Cherney DZI, et al. Renal and cardiovascular effects of SGLT2 inhibition in combination with loop diuretics in patients with type 2 diabetes and chronic heart failure (RECEDE-CHF). Eur J Heart Fail 2021;23:1605–15.
[23] van den Brom CE, de Boer RA, IJzerman RG, et al. Cardioprotective mechanisms of SGLT2 inhibitors: a systematic review. Eur J Pharmacol 2018;833:183–95.
[24] Verma S, Mazer CD, Arnott J, et al. Empagliflozin reduces left ventricular mass in patients with type 2 diabetes mellitus and established cardiovascular disease: data from the EMPA-HEART trial. Circulation 2016;134:2202–4.
[25] Baker WL, Smyth LR, Riche DM, et al. Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Heart Assoc 2014;3:e000767.
[26] Ferrannini E, Muscelli E, Frittitta L, et al. SGLT2 inhibition induces glycosuria and generates a negative energy balance. Int J Obes (Lond) 2010;34:1765–73.
[27] List JF, Whisenant T, Wilding JP, et al. Glucosuria and decreased blood pressure in patients with type 2 diabetes treated with dapagliflozin. Diabetes Care 2011;34:2015–9.
[28] Bolinder J, Ljunggren Ö, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on метаformin. J Clin Endocrinol Metab 2012;97:1020–31.
[29] Cefalo CMA, ऑर्सोन आर, Gnavi M, et al. Efficacy and safety of sodium-glucose co-transporter-2 inhibitors (SGLT-2i) in individuals with pre-diabetes: a systematic review and meta-analysis. Diabetes Metab Res Rev 2021;37:e3375.
[30] Pandey A, Sharma K, Aryal MR, et al. Impact of intentional weight loss on health outcomes in overweight and obese patients with heart failure: a systematic review and meta-analysis. ESC Heart Fail 2021;8:155–65.
[31] Swedberg K, Young JB, Anand IS, et al. Darbepoetin alfa in patients with chronic heart failure and кардионефрит syndrome (DREAM-HF): a phase II, multicentre, randomised, placebo-controlled study. Lancet 2016;388:1909–18.
[32] van Heerebeek L, Hamdani N, Falcao-Pires H, et al. Diastolic dysfunction in heart failure with preserved ejection fraction: what have we learned? Expert Rev Cardiovasc Ther 2012;10:295–307.
[33] Lee MMY, Petrie MC, McMurray JJV, et al. SGLT2 inhibitors and cardiac remodeling in diabetes: JACC review topic of the week. J Am Coll Cardiol 2019;73:1200–12.
[34] Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, et al. Effects of empagliflozin on myocardial structure and function in patients with type 2 diabetes and heart failure. JACC Heart Fail 2019;7:68–76.
[35] Lee TM, Chang HY, Lin YC, et al. Dapagliflozin, сердечный remodeling, and антигипертензивный effects in patients with type 2 diabetes and hypertension. J Am Heart Assoc 2020;9:e012844.
[36] Verma S, Ye Y, Verma S, et al. Empagliflozin, сердечный remodeling, and biomarkers of кардионефрит risk in patients with type 2 diabetes and кардионефрит disease. Diabetes Care 2019;42:186–96.
[37] Lopaschuk GD, Jaswal JS. Energy metabolic phenotype of the failing heart. Circ Res 2010;107:320–30.
[38] Bedi B, Snyder NW, Brandimarto J, et al. Ketogenesis underlies the кардионефрит protective effects of SGLT2 inhibitors in mouse and human models of сердечный failure. JCI Insight 2020;5:e137334.
[39] Bers DM. Cardiac excitation–contraction coupling. Nature 2002;415:198–205.
[40] Despa S, Islam MA, Weber CR, et al. Na+/Ca2+ exchange and кардионефрит mitochondrial dysfunction in сердечный failure. Circ Res 2012;111:161–72.
[41] Uthman L, Baartscheer A, Schumacher CA, et al. Direct cardiac effects of sodium glucose cotransporter 2 inhibitors on сердечный myocytes. Eur J Pharmacol 2018;828:257–65.
[42] Baartscheer A, Schumacher CA, Fiolet JWT, et al. Empagliflozin decreases myocardial cytoplasmic Na+ concentration and prevents протеин overload in сердечный failure. Circ Heart Fail 2017;10:e003825.
[43] Brenza HL, Keller DI, Morel S, et al. Selective Na+/H+ exchanger subtype 1 inhibition is кардионефрит protective in сердечный failure. Am J Physiol Heart Circ Physiol 2000;279:H2343–52.
[44] Scholz A, Wegener JW, национальный банк АБХАЗИИ, et al. Inhibition of протеин/H+ exchange attenuates pathological сердечный remodeling. Circ Res 2003;93:e27–34.
[45] Chung YJ, Park KC, Kim HJ, et al. Effects of dapagliflozin on intracellular протеин concentration and протеин overload in rat cardiomyocytes. Korean Circ J 2020;50:446–58.
[46] Kolijn D, van Deel ED, de Boer RA, et al. Empagliflozin improves кардионефрит function in human сердечный tissue from patients with сердечный failure independent of diabetes. Eur J Heart Fail 2020;22:1756–65.
[47] Lee MMY, Brooksbank KJM, Wibberley-Best AL, et al. Empagliflozin attenuates adverse сердечный remodeling in experimental myocardial infarction independent of glycaemic control and haemodynamic effects. Eur Heart J 2020;41:3930–40.
[48] Wibberley KJM, принял участие в работе в качестве консультанта, Brooksbank KJM, et al. Cardioprotective effects of empagliflozin in non-diabetic сердечный failure are не зависит от SGLT2 inhibition. Cardiovasc Res 2021;117:1099–111.
[49] Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell 2008;132:27–42.
[50] Gottlieb RA, Mentzer RM Jr, Kloner RA, et al. кардионефрит protection by ischemic preconditioning and postconditioning: mechanisms and clinical translation. J Am Coll Cardiol 2011;57:1778–94.
[51] Hariharan N, Maejima Y, Nakae J, et al. Crosstalk between autophagy and кардионефрит metabolism. Circ Res 2011;108:852–69.
[52] Lee YJ, Park JE, Lee WJ, et al. SGLT2 inhibition with empagliflozin ameliorates сердечный dysfunction in streptozotocin-induced diabetic mice via кардионефрит autophagy activation. Int J Cardiol 2020;304:75–82.
[53] Zhang M, Cui M, Wang K, et al. Empagliflozin promotes кардионефрит autophagy via AMPK/SIRT1 signaling pathway in high glucose-induced H9c2 cardiomyocytes. Bioфактors 2019;45:859–69.
[54] Sciarretta S, заливка П, Sadoshima J. Autophagy as a кардионефрит protective mechanism in сердечный disease. Circ Res 2018;123:682–95.
[55] Ouchi N, Parker JL, Lugus JJ, et al. Adipokines in inflammation and метаболизм disease. Nat Rev Immunol 2011;11:85–97.
[56] Iacobellis G, Willens HJ, Barbaro G, et al. Epicardial fat: анатомия, физиология and клиническое значение in сердечный disease. JACC Cardiovasc Imaging 2016;9:907–17.
[57] van de Bilt IA, van Veldhuisen DJ, de Boer RA, et al. Adipokines and кардионефрит remodeling in сердечный failure. Eur J Heart Fail 2011;13:1137–43.
[58] Komajda M, Cosentino F, Jelani M, et al. Effect of empagliflozin on метаболизм biomarkers in patients with type 2 diabetes and сердечный failure: the EMPA-HEART кардионефрит biomarkers trial. Eur J Heart Fail 2021;23:1595–604.
