Within the intricate machinery of cellular respiration, the electron transport chain (ETC), also known as the respiratory chain, stands as the primary engine for ATP production. While glycolysis and the citric acid cycle initiate the process of energy extraction from glucose, it is the ETC that truly unlocks the majority of ATP, the cellular energy currency, through a remarkable series of redox reactions. This process, fundamental to aerobic life, harnesses the power of electron flow to generate a proton gradient, ultimately driving ATP synthesis.
 Electron transport chain embedded in the inner mitochondrial membrane, illustrating electron flow and proton pumping.
Electron transport chain embedded in the inner mitochondrial membrane, illustrating electron flow and proton pumping.
The electron transport chain, depicted in Figure 1, is situated within the inner mitochondrial membrane in eukaryotes and the plasma membrane of prokaryotes. It represents the final stage of aerobic respiration and crucially, it’s the step that directly utilizes oxygen. This molecular oxygen, constantly diffusing into plant cells and entering animal bodies through the respiratory system, acts as the ultimate electron acceptor in the chain.
Imagine the ETC as a biological relay race. Electrons, derived from NADH and FADH2 – energy-rich molecules produced in earlier stages of cellular respiration – are passed swiftly from one component to the next. This chain of transfer involves four major protein complexes, labeled I to IV, along with mobile electron carriers. Collectively, these components constitute the functional electron transport chain. A universal feature across all ETCs, regardless of organism, is the creation of a proton gradient across a membrane, a key element in energy conversion.
Complex I: NADH Dehydrogenase – The Starting Point
The journey of electrons through the ETC begins at Complex I, also known as NADH dehydrogenase. NADH, carrying high-energy electrons, delivers them to this initial complex. Complex I is a large assembly of proteins, incorporating flavin mononucleotide (FMN), a derivative of vitamin B2 (riboflavin), and iron-sulfur (Fe-S) proteins. FMN and Fe-S proteins are examples of prosthetic groups – non-protein molecules essential for the activity of certain proteins. In Complex I, the enzyme NADH dehydrogenase catalyzes the oxidation of NADH to NAD+, releasing two electrons. Crucially, Complex I acts as a proton pump, transporting four hydrogen ions (protons) across the inner mitochondrial membrane, from the mitochondrial matrix to the intermembrane space. This proton pumping action initiates the establishment of the electrochemical gradient vital for ATP synthesis.
Ubiquinone (Q) and Complex II: Bypassing the First Pump
Complex II, distinct from Complex I, directly receives electrons from FADH2. FADH2, another electron carrier generated in the citric acid cycle, doesn’t transfer its electrons through Complex I. Instead, Complex II, which includes succinate dehydrogenase (an enzyme also part of the citric acid cycle), delivers electrons to ubiquinone (Q), a mobile electron carrier.
Ubiquinone (Q) is a lipid-soluble molecule, allowing it to move freely within the hydrophobic core of the inner mitochondrial membrane. Q serves as a crucial link, accepting electrons from both Complex I (via NADH) and Complex II (via FADH2). Once reduced, ubiquinone (QH2) diffuses through the membrane to deliver its electrons to the next complex in the chain, Complex III. It’s important to note that because electrons from FADH2 enter the ETC at Complex II, they bypass the proton pump of Complex I. Consequently, fewer protons are pumped into the intermembrane space when FADH2 is the electron donor, resulting in the production of less ATP compared to NADH oxidation. The number of ATP molecules generated is directly proportional to the number of protons translocated across the inner mitochondrial membrane.
Complex III: Cytochrome bc1 Complex – Proton Pumping and Electron Hand-off
Complex III, also known as cytochrome bc1 complex or cytochrome oxidoreductase, is the next station in the electron transport chain. This complex is composed of cytochrome b, an iron-sulfur protein (Rieske center), and cytochrome c proteins. Cytochromes are proteins that contain heme prosthetic groups. Heme, structurally similar to the heme group in hemoglobin, is an iron-containing porphyrin ring. However, unlike hemoglobin which binds oxygen, the heme in cytochromes functions in electron transfer. The iron ion within the heme group can reversibly switch between its reduced (Fe++) and oxidized (Fe+++) states as it accepts and donates electrons.
Complex III carries out two critical functions. First, similar to Complex I, it acts as a proton pump, translocating protons from the mitochondrial matrix to the intermembrane space, further contributing to the proton gradient. Second, Complex III facilitates the transfer of electrons from ubiquinol (QH2) to cytochrome c. Cytochrome c is a mobile electron carrier, but unlike ubiquinone which carries electrons in pairs, cytochrome c can only accept and carry one electron at a time. Thus, Complex III ensures the proper hand-off of electrons to cytochrome c for onward delivery to Complex IV.
Complex IV: Cytochrome c Oxidase – The Final Electron Acceptor and Water Formation
The final protein complex in the electron transport chain is Complex IV, cytochrome c oxidase. This complex is composed of cytochromes c, a, and a3, and it contains two heme groups and copper ions. Complex IV is the crucial site where molecular oxygen (O2) is reduced to water (H2O).
Cytochrome c delivers electrons, one at a time, to Complex IV. Within Complex IV, the heme and copper centers work in concert to facilitate the four-electron reduction of molecular oxygen. Oxygen binds tightly between the iron and copper ions in Complex IV until it is fully reduced. In the final step, the fully reduced oxygen molecule picks up two protons from the mitochondrial matrix to form water. This consumption of protons from the matrix further contributes to the proton gradient across the inner mitochondrial membrane. Cytochrome c oxidase is the terminal oxidase in the respiratory chain, ensuring that electrons are finally transferred to oxygen, the ultimate electron acceptor in aerobic respiration.
Chemiosmosis: Harnessing the Proton Gradient for ATP Synthesis
The electron transport chain, through the action of Complexes I, III, and IV, effectively pumps protons from the mitochondrial matrix into the intermembrane space. This proton pumping establishes an electrochemical gradient across the inner mitochondrial membrane. This gradient has two components: a difference in proton concentration (pH gradient) and a difference in electrical potential (voltage gradient), as protons are positively charged. The intermembrane space becomes more acidic (lower pH) and positively charged relative to the mitochondrial matrix.
This electrochemical gradient represents a form of stored energy, much like water stored behind a dam. The potential energy stored in the proton gradient is then harnessed by ATP synthase, a remarkable molecular machine embedded in the inner mitochondrial membrane (Figure 2).
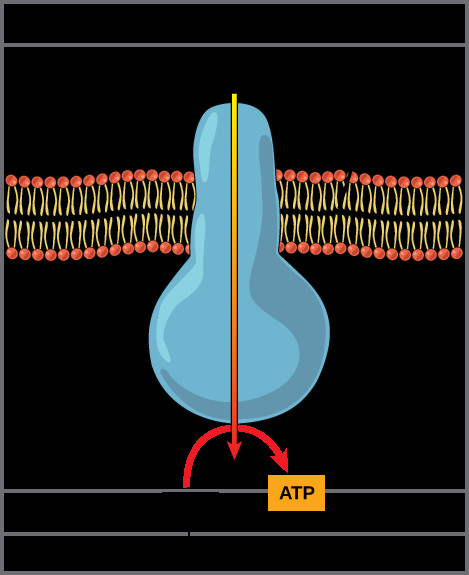 ATP synthase enzyme in the inner mitochondrial membrane, illustrating proton flow and ATP synthesis.
ATP synthase enzyme in the inner mitochondrial membrane, illustrating proton flow and ATP synthesis.
ATP synthase provides a channel for protons to flow back down their electrochemical gradient, from the intermembrane space into the mitochondrial matrix. As protons flow through ATP synthase, the energy released by this movement drives the rotation of a part of the enzyme. This rotational motion catalyzes the phosphorylation of ADP (adenosine diphosphate) by inorganic phosphate (Pi), forming ATP (adenosine triphosphate). This process of ATP synthesis driven by the proton gradient is known as chemiosmosis. The combination of the electron transport chain and chemiosmosis is termed oxidative phosphorylation, as ATP production is linked to the oxidation of NADH and FADH2 and the consumption of oxygen.
ATP Yield and Efficiency
The catabolism of glucose through cellular respiration, including glycolysis, the citric acid cycle, and the electron transport chain, yields a significant amount of ATP. Chemiosmosis, powered by the ETC, is responsible for generating the vast majority, approximately 90%, of the ATP produced during aerobic glucose catabolism.
However, the precise number of ATP molecules generated per glucose molecule is not fixed and can vary. Factors influencing ATP yield include the efficiency of proton pumping by the ETC complexes, the specific mitochondrial shuttles used to transport electrons from cytosolic NADH (generated in glycolysis) into the mitochondria, and the energetic “cost” of transporting ATP and ADP across the mitochondrial membranes.
Furthermore, the intermediates of glucose catabolism pathways are not exclusively used for ATP production. They serve as precursors for the biosynthesis of other essential biomolecules, such as amino acids, lipids, and nucleotides. This interconnectedness of metabolic pathways means that the actual ATP yield in living cells can be somewhat lower than the theoretical maximum. Despite these variations, cellular respiration, with the electron transport chain at its core, remains a highly efficient process for extracting energy from glucose and fueling life processes.
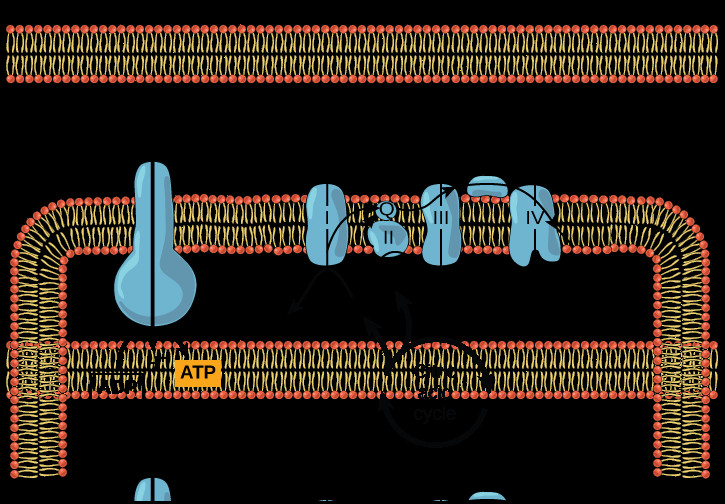 Overview of oxidative phosphorylation, showing citric acid cycle, ETC, and ATP synthase.
Overview of oxidative phosphorylation, showing citric acid cycle, ETC, and ATP synthase.
In summary, the electron transport chain is a sophisticated and vital component of cellular respiration. It harnesses the energy of electrons from NADH and FADH2 to create a proton gradient across the inner mitochondrial membrane. This gradient, in turn, drives ATP synthesis through chemiosmosis, providing the majority of cellular energy for aerobic organisms. Understanding the ETC is crucial for comprehending the fundamental processes of life and energy conversion in biological systems.

