Cellular respiration is the fundamental process that fuels life, converting the energy stored in nutrients into a usable form of energy for cells: ATP (adenosine triphosphate). While glycolysis and the citric acid cycle are crucial initial steps in this energy production, the vast majority of ATP generated during aerobic respiration comes from a remarkable process known as the electron transport chain (ETC), also referred to as the respiratory chain. This intricate system, embedded within the mitochondria of eukaryotic cells and the plasma membrane of prokaryotes, is a series of protein complexes that orchestrate a flow of electrons, ultimately leading to the synthesis of ATP.
 Electron transport chain embedded in the inner mitochondrial membrane, illustrating electron flow and proton pumping.
Electron transport chain embedded in the inner mitochondrial membrane, illustrating electron flow and proton pumping.
Unpacking the Electron Transport Chain: A Step-by-Step Guide
The electron transport chain is the final stage of aerobic respiration and is the only part of glucose metabolism that directly utilizes oxygen. Imagine it as a biological “bucket brigade” where electrons are passed sequentially from one component to the next. This series of redox reactions (reduction and oxidation) doesn’t just move electrons; it harnesses the energy released during these transfers to pump protons (hydrogen ions, H+) across a membrane, creating an electrochemical gradient. This gradient then powers the synthesis of ATP, the cell’s energy currency.
The ETC is composed of four major protein complexes, labeled I to IV, along with mobile electron carriers. In eukaryotes, these complexes reside in the inner mitochondrial membrane, while in prokaryotes, they are located in the plasma membrane. Let’s delve into each component to understand how this process works.
Complex I: NADH-CoQ Reductase (or NADH Dehydrogenase)
The ETC journey begins at Complex I, also known as NADH-CoQ reductase. This complex receives electrons from NADH (nicotinamide adenine dinucleotide), a crucial electron carrier generated during glycolysis and the citric acid cycle. NADH delivers two high-energy electrons to Complex I, oxidizing NADH back to NAD+, which can then be reused in earlier stages of respiration.
Complex I is a massive protein containing flavin mononucleotide (FMN) and iron-sulfur (Fe-S) clusters. FMN, derived from vitamin B2 (riboflavin), acts as a prosthetic group, a non-protein molecule essential for protein function. As electrons move through Complex I, energy is released, which is used to pump four protons from the mitochondrial matrix to the intermembrane space. This proton pumping is the first contribution to the crucial proton gradient.
Complex II: Succinate-CoQ Reductase (or Succinate Dehydrogenase)
Complex II, also called succinate-CoQ reductase or succinate dehydrogenase, takes a different entry point into the ETC. It receives electrons from FADH2 (flavin adenine dinucleotide), another electron carrier produced in the citric acid cycle. FADH2 is oxidized to FAD, releasing two electrons that enter Complex II.
Interestingly, Complex II does not pump protons across the membrane. This means that electrons entering through FADH2 contribute less to the proton gradient and ultimately result in slightly less ATP production compared to electrons from NADH. Complex II includes iron-sulfur (Fe-S) proteins and directly interacts with ubiquinone (Q), passing electrons to the next stage.
Coenzyme Q (Ubiquinone): The Mobile Electron Carrier
Ubiquinone (Q), often simply referred to as coenzyme Q, is a small, lipid-soluble molecule that acts as a mobile electron carrier within the inner mitochondrial membrane. It shuttles electrons from both Complex I and Complex II to Complex III.
Q is unique because it can accept electrons from both NADH (via Complex I) and FADH2 (via Complex II). Once Q accepts electrons, it becomes reduced to QH2 (ubiquinol). QH2 then diffuses through the membrane to deliver its electrons to Complex III.
Complex III: CoQ-Cytochrome c Reductase (or Cytochrome bc1 complex)
Complex III, also known as CoQ-cytochrome c reductase or cytochrome bc1 complex, receives electrons from QH2. This complex contains cytochromes, proteins with heme prosthetic groups. Heme is similar to the heme in hemoglobin but, instead of carrying oxygen, it carries electrons. The iron ion at the center of heme cycles between a reduced state (Fe++) and an oxidized state (Fe+++) as it participates in electron transfer.
Complex III plays a vital role in proton pumping. As electrons are transferred through Complex III, four protons are pumped from the mitochondrial matrix into the intermembrane space – two protons are chemically removed from the matrix and another two are transported across the membrane. Complex III then passes electrons to another mobile electron carrier, cytochrome c.
Cytochrome c: Another Mobile Electron Carrier
Cytochrome c is a small, water-soluble protein located in the intermembrane space. It acts as another mobile electron carrier, accepting electrons one at a time from Complex III and transporting them to Complex IV. Unlike ubiquinone, cytochrome c can only carry a single electron at a time, highlighting the intricate steps involved in electron flow.
Complex IV: Cytochrome c Oxidase
Complex IV, known as cytochrome c oxidase, is the final protein complex in the electron transport chain. It receives electrons from cytochrome c. This complex is crucial because it is where the final electron acceptor, oxygen, comes into play.
Complex IV contains cytochromes a and a3, as well as copper ions. It meticulously facilitates the reduction of molecular oxygen (O2) to water (H2O). In this process, oxygen tightly binds to Complex IV until it is fully reduced by accepting electrons. This reduction of oxygen also requires hydrogen ions from the mitochondrial matrix, further contributing to the proton gradient across the inner mitochondrial membrane, and Complex IV itself pumps protons – contributing to a total of two protons pumped across the membrane per pair of electrons. The formation of water is a critical step, effectively removing the “spent” electrons from the system and allowing the ETC to continue operating.
Chemiosmosis: Harnessing the Proton Gradient for ATP Synthesis
The electron transport chain, through Complexes I, III, and IV, diligently pumps protons from the mitochondrial matrix into the intermembrane space. This pumping action creates an electrochemical gradient – a difference in both proton concentration (pH gradient) and electrical charge across the inner mitochondrial membrane. The intermembrane space becomes more acidic (higher H+ concentration) and positively charged compared to the mitochondrial matrix.
This gradient represents stored potential energy, much like water held behind a dam. The protons “want” to move back down their concentration and charge gradient into the matrix. However, the inner mitochondrial membrane is generally impermeable to protons. The only pathway for protons to re-enter the matrix is through a remarkable protein complex called ATP synthase.
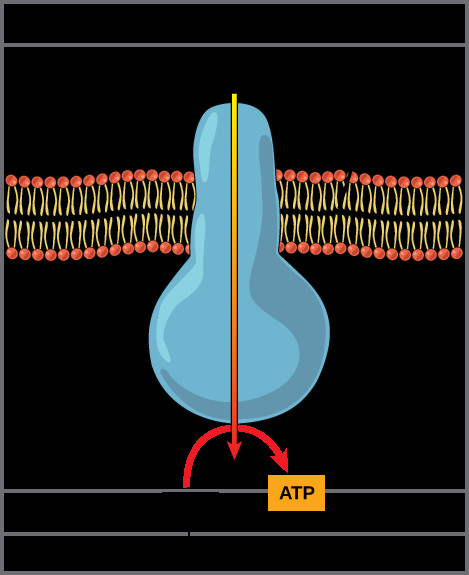 ATP synthase enzyme embedded in the inner mitochondrial membrane, showing proton flow and ATP synthesis.
ATP synthase enzyme embedded in the inner mitochondrial membrane, showing proton flow and ATP synthesis.
ATP synthase acts as a molecular turbine. As protons flow down their electrochemical gradient through ATP synthase, the energy released from this movement is used to drive the rotation of a part of the enzyme. This rotation catalyzes the phosphorylation of ADP (adenosine diphosphate) by adding an inorganic phosphate (Pi), forming ATP. This process, coupling the electron transport chain and proton gradient to ATP synthesis, is called chemiosmosis or oxidative phosphorylation. Chemiosmosis is responsible for generating the vast majority (approximately 90%) of ATP during aerobic cellular respiration.
Factors Affecting ATP Yield and Efficiency
While theoretically, the oxidation of one molecule of glucose can yield a significant amount of ATP, the actual yield in living cells is often lower and can vary. Several factors influence the efficiency of ATP production via the electron transport chain:
- Proton Pumping Efficiency: The number of protons pumped by Complexes I, III, and IV can vary slightly between species and conditions, affecting the proton gradient strength and thus ATP yield.
- Electron Shuttle Systems: NADH generated during glycolysis in the cytoplasm cannot directly enter the mitochondria in eukaryotes. Electrons from cytosolic NADH are shuttled into the mitochondria using different shuttle systems. Some shuttles deliver electrons to mitochondrial NAD+ (yielding more ATP), while others deliver electrons to mitochondrial FAD (yielding less ATP). For instance, the malate-aspartate shuttle is more efficient than the glycerol-3-phosphate shuttle.
- Metabolic Intermediates and Diversion: Intermediates of glycolysis and the citric acid cycle are not solely dedicated to ATP production. They can be diverted into other metabolic pathways for biosynthesis of amino acids, nucleotides, lipids, and other essential molecules. This diversion reduces the amount of substrate available for the ETC and ATP synthesis.
- Proton Leakage and Uncoupling: The inner mitochondrial membrane is not perfectly impermeable to protons. Some protons can leak back into the matrix without passing through ATP synthase, reducing the efficiency of ATP production. Uncoupling agents, like dinitrophenol (DNP), can dramatically increase proton leakage, disrupting the proton gradient and reducing ATP synthesis, while paradoxically increasing heat production as energy is released as heat instead of being captured in ATP.
Clinical Relevance and Implications
The electron transport chain is not just a biochemical pathway; it is essential for life, and its dysfunction can have severe consequences. Several toxins and genetic disorders target the ETC, highlighting its clinical significance:
- Cyanide Poisoning: Cyanide is a potent inhibitor of cytochrome c oxidase (Complex IV). By blocking Complex IV, cyanide halts electron transport and ATP production. This leads to rapid cellular energy depletion and can be fatal.
- Dinitrophenol (DNP): As mentioned earlier, DNP is an uncoupling agent that increases proton permeability across the inner mitochondrial membrane. While initially used as a weight-loss drug due to reduced ATP production and increased metabolism, its use is extremely dangerous due to the risk of hyperthermia and other severe side effects.
- Mitochondrial Diseases: A range of genetic disorders can affect different components of the electron transport chain. These mitochondrial diseases can manifest in various ways, often affecting tissues with high energy demands, such as muscles and nerves, leading to muscle weakness, neurological problems, and other serious health issues.
In Summary: The Electron Transport Chain as the Cellular Powerhouse
The electron transport chain is a remarkable and essential process that underpins aerobic life. By orchestrating a series of redox reactions and proton pumping, it creates an electrochemical gradient that drives ATP synthesis through chemiosmosis. This intricate system, composed of protein complexes and mobile electron carriers, effectively extracts energy from electron carriers like NADH and FADH2, ultimately reducing oxygen to water and generating the vast majority of ATP needed to power cellular activities. Understanding the electron transport chain is crucial for comprehending cellular metabolism, energy production, and the basis of many physiological and pathological conditions.
Figure References:
