Cellular respiration is the fundamental process that fuels life, converting the energy stored in glucose and other organic molecules into a usable form of energy, ATP (adenosine triphosphate). While glycolysis and the citric acid cycle are crucial initial steps in this energy extraction, the majority of ATP production occurs in the final stage: the electron transport chain (ETC). Often visualized and explained through a Diagram Electron Transport, this intricate system is the cornerstone of aerobic respiration. This article delves into the complexities of the electron transport chain, elucidating its components, function, and vital role in generating the energy that powers our cells.
 Diagram of the electron transport chain, illustrating the four protein complexes (I-IV) within the inner mitochondrial membrane, electron flow from NADH and FADH2 to oxygen, proton pumping, and ATP production during cellular respiration.
Diagram of the electron transport chain, illustrating the four protein complexes (I-IV) within the inner mitochondrial membrane, electron flow from NADH and FADH2 to oxygen, proton pumping, and ATP production during cellular respiration.
What is the Electron Transport Chain?
The electron transport chain, also known as the respiratory chain, is a series of protein complexes embedded within the inner mitochondrial membrane of eukaryotic cells and the plasma membrane of prokaryotes. It acts as the final pathway in aerobic respiration, utilizing oxygen as the ultimate electron acceptor. Imagine it as a carefully orchestrated relay race where electrons, derived from NADH and FADH2 generated in earlier stages of cellular respiration, are passed sequentially from one carrier molecule to the next.
This process is not merely a simple transfer of electrons; it’s a series of redox reactions, where one molecule is oxidized (loses electrons) and another is reduced (gains electrons). As electrons move down the chain, a small amount of energy is released at each step. This carefully harvested energy is not directly used to produce ATP, but rather to pump protons (H+) from the mitochondrial matrix to the intermembrane space. This pumping action creates an electrochemical gradient, a crucial prerequisite for the final act of ATP synthesis.
Components of the Electron Transport Chain
The electron transport chain is composed of five major components:
-
Complex I (NADH-Q Oxidoreductase): This massive complex, also known as NADH dehydrogenase, is the entry point for electrons from NADH. It contains flavin mononucleotide (FMN), a derivative of vitamin B2, and iron-sulfur (Fe-S) protein clusters. Complex I oxidizes NADH back to NAD+, releasing two electrons that are passed to coenzyme Q (ubiquinone). Importantly, Complex I also functions as a proton pump, translocating four protons across the inner mitochondrial membrane for every pair of electrons transferred.
-
Complex II (Succinate-Q Oxidoreductase): Complex II is the entry point for electrons from FADH2. It contains succinate dehydrogenase, an enzyme from the citric acid cycle, and also utilizes iron-sulfur (Fe-S) centers. FADH2 is oxidized to FAD, and its electrons are passed to coenzyme Q. Unlike Complex I, Complex II does not pump protons across the membrane.
-
Coenzyme Q (Ubiquinone): Often simply referred to as Q, ubiquinone is a small, lipid-soluble molecule that is not a protein but a crucial mobile electron carrier. It resides within the hydrophobic core of the inner mitochondrial membrane, accepting electrons from both Complex I and Complex II. Upon accepting electrons, Q becomes reduced to QH2 (ubiquinol) and diffuses through the membrane to deliver electrons to Complex III.
-
Complex III (Q-cytochrome c Oxidoreductase): Also known as the cytochrome bc1 complex, Complex III accepts electrons from QH2. It contains cytochromes (cytochrome b and cytochrome c1) and iron-sulfur proteins (Rieske iron-sulfur protein). Cytochromes are proteins that contain a heme group, similar to the heme in hemoglobin, but instead of carrying oxygen, the iron atom in heme cycles between Fe2+ (reduced) and Fe3+ (oxidized) states as it carries electrons. Complex III facilitates the transfer of electrons from ubiquinol to cytochrome c, another mobile electron carrier, and simultaneously pumps protons across the membrane into the intermembrane space.
-
Cytochrome c: Cytochrome c is a small, water-soluble protein located in the intermembrane space. It acts as a mobile electron carrier, accepting electrons one at a time from Complex III and delivering them to Complex IV.
-
Complex IV (Cytochrome c Oxidase): This final complex, cytochrome c oxidase, is the terminal electron acceptor of the chain. It contains cytochromes a and a3, and copper ions. Complex IV receives electrons from cytochrome c and ultimately transfers them to molecular oxygen (O2), the final electron acceptor in aerobic respiration. In this process, oxygen is reduced to water (H2O). Complex IV also pumps protons across the membrane, contributing to the proton gradient.
Chemiosmosis and ATP Synthase: Harnessing the Proton Gradient
The electron transport chain, through the action of Complexes I, III, and IV, establishes a significant proton gradient across the inner mitochondrial membrane. The intermembrane space becomes more acidic (higher concentration of H+) compared to the mitochondrial matrix. This gradient represents stored potential energy, much like water held behind a dam.
The crucial enzyme that harnesses this potential energy is ATP synthase (Complex V), a remarkable molecular machine also embedded in the inner mitochondrial membrane. ATP synthase provides a channel for protons to flow down their electrochemical gradient, back into the mitochondrial matrix. This flow of protons drives the rotation of a part of ATP synthase, which in turn catalyzes the phosphorylation of ADP (adenosine diphosphate) to ATP. This process, where ATP is generated using the energy of a proton gradient, is called chemiosmosis or oxidative phosphorylation. It is responsible for the vast majority of ATP produced during aerobic respiration.
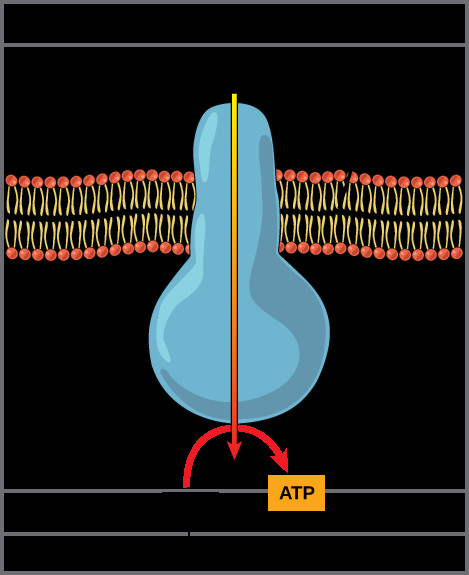 Illustration of ATP synthase, a molecular machine in the mitochondrial membrane, showing proton flow from the intermembrane space to the matrix powering ATP synthesis from ADP and inorganic phosphate.
Illustration of ATP synthase, a molecular machine in the mitochondrial membrane, showing proton flow from the intermembrane space to the matrix powering ATP synthesis from ADP and inorganic phosphate.
ATP Yield and Efficiency
The theoretical maximum yield of ATP from one molecule of glucose through aerobic respiration is estimated to be around 36-38 ATP molecules in eukaryotes. However, the actual yield in living cells is often lower, closer to 30-32 ATP. This variability arises from several factors, including:
- Proton leaks: The inner mitochondrial membrane is not perfectly impermeable to protons, and some protons may leak back into the matrix without passing through ATP synthase, reducing the efficiency of ATP production.
- Shuttle systems: NADH produced during glycolysis in the cytoplasm cannot directly enter the mitochondria in eukaryotes. Shuttle systems are required to transport the electrons into the mitochondria, and these systems can result in the use of either NAD+ or FAD+ as electron carriers within the mitochondria. FADH2, entering at Complex II, leads to the pumping of fewer protons compared to NADH entering at Complex I, resulting in slightly less ATP production.
- Metabolic intermediates used for other pathways: Intermediates from glycolysis and the citric acid cycle are not solely dedicated to ATP production; they can be diverted into other biosynthetic pathways to create amino acids, nucleotides, lipids, and other essential molecules.
Despite these factors, the electron transport chain and oxidative phosphorylation remain remarkably efficient in energy conversion. Approximately 34% of the energy stored in a glucose molecule is captured as ATP through cellular respiration.
The Importance of the Electron Transport Chain
The electron transport chain is indispensable for aerobic life. It is the primary mechanism by which cells extract the majority of energy from food molecules. Without a functional electron transport chain, cells would be limited to the relatively inefficient process of glycolysis for ATP production, which is insufficient to support the energy demands of complex organisms.
Furthermore, the electron transport chain plays a crucial role in:
- Regenerating electron carriers: By oxidizing NADH and FADH2 back to NAD+ and FAD, the ETC ensures a continuous supply of these electron carriers for glycolysis and the citric acid cycle to proceed.
- Metabolic flexibility: The intermediates of cellular respiration, generated before the ETC, serve as building blocks for various other biomolecules, highlighting the interconnectedness of metabolic pathways.
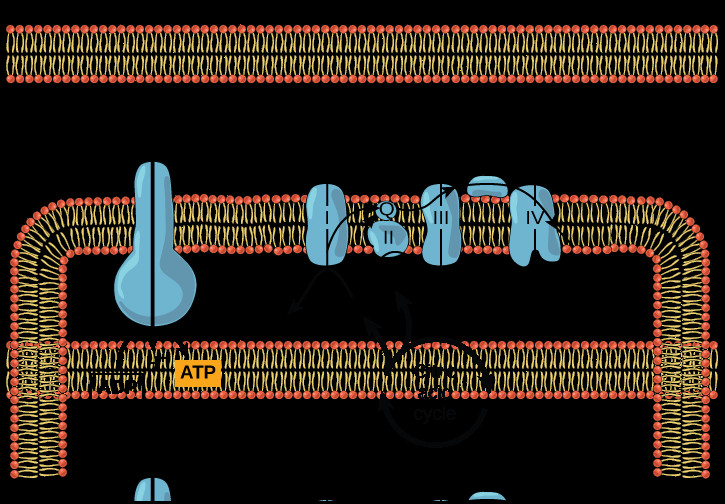 Comprehensive diagram of oxidative phosphorylation, depicting the citric acid cycle supplying NADH and FADH2 to the electron transport chain, proton gradient formation across the mitochondrial membrane, and ATP production by ATP synthase.
Comprehensive diagram of oxidative phosphorylation, depicting the citric acid cycle supplying NADH and FADH2 to the electron transport chain, proton gradient formation across the mitochondrial membrane, and ATP production by ATP synthase.
Conclusion
The electron transport chain, often represented visually in a diagram electron transport, is a marvel of biological engineering. This intricate series of protein complexes and mobile carriers efficiently harvests energy from electrons to create a proton gradient, which is then elegantly utilized by ATP synthase to generate the energy currency of the cell – ATP. Understanding the electron transport chain is fundamental to comprehending cellular respiration and the energetic basis of life itself. Its complexity and efficiency underscore its critical importance for all aerobic organisms.
