Cellular respiration is a fundamental process of life, converting nutrients into usable energy in the form of ATP (adenosine triphosphate). While glycolysis and the citric acid cycle play crucial roles in the initial stages of this energy production, the majority of ATP is generated through the electron transport chain (ETC), also known as the respiratory chain. To truly grasp this vital process, a Diagram Electron Transport Chain becomes an indispensable tool. This article delves into the intricacies of the electron transport chain, elucidating its components, mechanism, and significance in aerobic respiration, with a focus on understanding and interpreting the diagram electron transport chain.
Understanding the Electron Transport Chain and its Role
The electron transport chain is the final stage of aerobic respiration, occurring in the inner mitochondrial membrane of eukaryotes and the plasma membrane of prokaryotes. It’s a series of protein complexes and organic molecules that facilitate a sequence of redox reactions, ultimately leading to the production of ATP. As you can see in the diagram electron transport chain (Figure 1), this intricate system accepts electrons from electron carriers generated in earlier stages of respiration and passes them down a chain, releasing energy in a controlled manner. This released energy is not directly used to make ATP, but rather to pump protons (H+) across the inner mitochondrial membrane, creating an electrochemical gradient.
 Diagram of the electron transport chain embedded in the inner mitochondrial membrane, illustrating the four complexes, coenzyme Q, cytochrome c, and the flow of electrons from NADH and FADH2 to molecular oxygen, coupled with proton pumping into the intermembrane space.Figure 1. Diagram electron transport chain: This illustration provides a visual representation of the electron transport chain, a series of electron transporters embedded within the inner mitochondrial membrane. It clearly depicts how electrons are shuttled from NADH and FADH2 to molecular oxygen through Complexes I-IV. The diagram also highlights the crucial process of proton pumping from the mitochondrial matrix to the intermembrane space, and the reduction of oxygen to water.
Diagram of the electron transport chain embedded in the inner mitochondrial membrane, illustrating the four complexes, coenzyme Q, cytochrome c, and the flow of electrons from NADH and FADH2 to molecular oxygen, coupled with proton pumping into the intermembrane space.Figure 1. Diagram electron transport chain: This illustration provides a visual representation of the electron transport chain, a series of electron transporters embedded within the inner mitochondrial membrane. It clearly depicts how electrons are shuttled from NADH and FADH2 to molecular oxygen through Complexes I-IV. The diagram also highlights the crucial process of proton pumping from the mitochondrial matrix to the intermembrane space, and the reduction of oxygen to water.
The diagram electron transport chain in Figure 1 effectively illustrates the key components: four protein complexes (Complex I, II, III, and IV) and mobile electron carriers (coenzyme Q and cytochrome c). Electrons derived from NADH and FADH2, produced during glycolysis and the citric acid cycle, enter the chain. NADH donates electrons to Complex I, while FADH2 donates electrons to Complex II. As electrons move through these complexes, protons are pumped from the mitochondrial matrix to the intermembrane space, against their concentration gradient. The final electron acceptor in this chain is molecular oxygen, which gets reduced to water, a crucial step in aerobic respiration.
Deconstructing the Diagram Electron Transport Chain: Key Components
To fully understand the diagram electron transport chain, let’s break down each component and its function as visualized in Figure 1.
Complex I: NADH-CoQ Reductase
Complex I, also known as NADH dehydrogenase, is the entry point for electrons from NADH. As depicted in the diagram electron transport chain, NADH transfers two electrons to Complex I. This complex contains flavin mononucleotide (FMN) and iron-sulfur (Fe-S) centers. The energy released during electron transfer is utilized by Complex I to pump four protons across the inner mitochondrial membrane, contributing significantly to the proton gradient. This proton pumping action is clearly indicated in most diagram electron transport chain representations.
Complex II: Succinate-CoQ Reductase
Complex II, or succinate dehydrogenase, is unique as it directly receives FADH2, bypassing Complex I. As shown in the diagram electron transport chain, FADH2 delivers its electrons to Complex II, which also contains Fe-S centers and FAD. While Complex II facilitates electron transfer to coenzyme Q, it does not pump protons across the membrane. This is why FADH2 contributes to slightly less ATP production compared to NADH. Careful observation of a diagram electron transport chain will highlight this difference in proton pumping capability between Complex I and Complex II.
Coenzyme Q (Ubiquinone)
Coenzyme Q (CoQ), also known as ubiquinone, is a mobile electron carrier. The diagram electron transport chain shows CoQ positioned between Complexes I & II and Complex III. It accepts electrons from both Complex I and Complex II, becoming reduced to QH2. Being lipid-soluble, CoQ diffuses freely within the inner mitochondrial membrane, effectively shuttling electrons from Complexes I & II to Complex III. Its role as a mobile carrier is crucial for connecting different parts of the electron transport chain, as visualized in any comprehensive diagram electron transport chain.
Complex III: CoQ-Cytochrome c Reductase
Complex III, also known as cytochrome bc1 complex or CoQ-cytochrome c reductase, receives electrons from reduced CoQ (QH2). As illustrated in the diagram electron transport chain, Complex III contains cytochromes (cytochrome b and cytochrome c1) and Fe-S proteins. Electron transfer through Complex III is coupled with the pumping of protons across the membrane. Typically, Complex III pumps four protons into the intermembrane space for every pair of electrons passed. The diagram electron transport chain often uses arrows or symbols to indicate this proton translocation. Complex III then passes electrons to cytochrome c.
Cytochrome c
Cytochrome c is another mobile electron carrier, but unlike CoQ, it is a protein and is water-soluble, residing in the intermembrane space. The diagram electron transport chain shows cytochrome c accepting electrons from Complex III and transferring them to Complex IV. Cytochrome c can only carry one electron at a time, in contrast to CoQ which carries two. This sequential electron transfer is a key feature depicted in detailed diagram electron transport chain illustrations.
Complex IV: Cytochrome c Oxidase
Complex IV, or cytochrome c oxidase, is the final protein complex in the electron transport chain. As indicated in the diagram electron transport chain, Complex IV receives electrons from cytochrome c. It contains cytochromes a and a3, and copper ions. Complex IV catalyzes the final redox reaction: the reduction of molecular oxygen (O2) to water (H2O). This step is essential for aerobic respiration, as oxygen acts as the terminal electron acceptor. The diagram electron transport chain clearly shows oxygen as the final destination of electrons. Furthermore, Complex IV also pumps protons across the membrane, though the number is variable, typically two protons per pair of electrons.
Chemiosmosis: Harnessing the Proton Gradient for ATP Synthesis
The pumping of protons by Complexes I, III, and IV creates an electrochemical gradient across the inner mitochondrial membrane. This gradient, with a higher concentration of protons in the intermembrane space and lower in the matrix, stores potential energy, much like water behind a dam. This process is known as chemiosmosis.
As depicted in Figure 2, protons flow back down their electrochemical gradient into the mitochondrial matrix through a channel in ATP synthase, a complex enzyme embedded in the inner mitochondrial membrane. The diagram electron transport chain and associated diagrams of ATP synthase (Figure 2) often illustrate this proton flow.
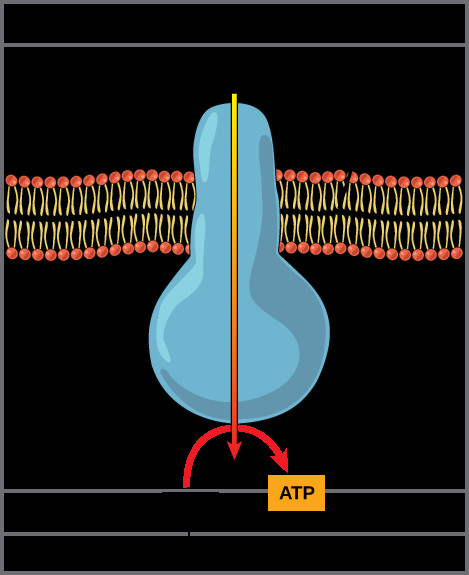 Diagram of ATP synthase enzyme in the inner mitochondrial membrane, showing proton movement from the intermembrane space to the mitochondrial matrix, driving ATP synthesis from ADP and inorganic phosphate.Figure 2. Diagram of ATP synthase: This diagram illustrates the ATP synthase enzyme, a molecular machine embedded in the inner mitochondrial membrane. It demonstrates how the flow of protons from an area of high concentration (intermembrane space) to low concentration (mitochondrial matrix) provides the energy to synthesize ATP from ADP and inorganic phosphate. Understanding this diagram is crucial for grasping the chemiosmotic mechanism.
Diagram of ATP synthase enzyme in the inner mitochondrial membrane, showing proton movement from the intermembrane space to the mitochondrial matrix, driving ATP synthesis from ADP and inorganic phosphate.Figure 2. Diagram of ATP synthase: This diagram illustrates the ATP synthase enzyme, a molecular machine embedded in the inner mitochondrial membrane. It demonstrates how the flow of protons from an area of high concentration (intermembrane space) to low concentration (mitochondrial matrix) provides the energy to synthesize ATP from ADP and inorganic phosphate. Understanding this diagram is crucial for grasping the chemiosmotic mechanism.
ATP synthase (Figure 2) acts like a molecular turbine. The energy from the proton flow drives the rotation of a part of ATP synthase, which in turn catalyzes the phosphorylation of ADP to ATP. This process, known as oxidative phosphorylation, is the major source of ATP in aerobic respiration. Figure 3 provides a broader context, showing how the electron transport chain and ATP synthase work in conjunction with the citric acid cycle.
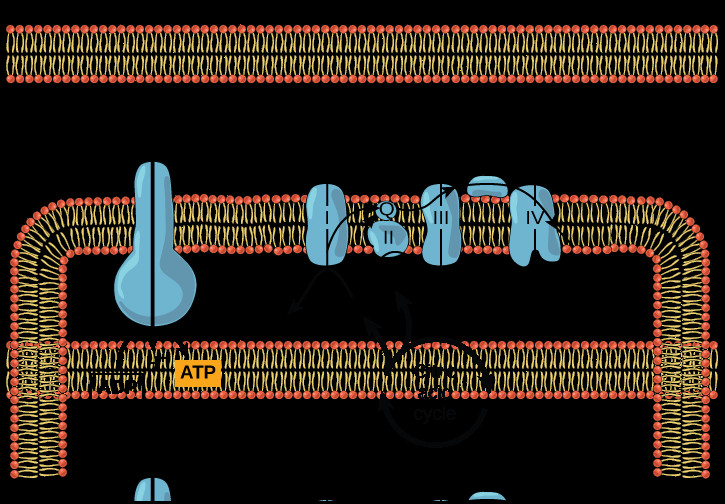 Diagram showing the electron transport chain and ATP synthase in the inner mitochondrial membrane, alongside the citric acid cycle in the mitochondrial matrix, illustrating the interconnectedness of these processes in ATP production.Figure 3. Diagram of Oxidative Phosphorylation: This diagram provides a comprehensive overview of oxidative phosphorylation, showing the citric acid cycle providing NADH and FADH2 to the electron transport chain. It illustrates how the ETC pumps protons to create a gradient, which ATP synthase then utilizes to generate ATP. This diagram effectively summarizes the entire process of ATP generation through the electron transport chain and chemiosmosis.
Diagram showing the electron transport chain and ATP synthase in the inner mitochondrial membrane, alongside the citric acid cycle in the mitochondrial matrix, illustrating the interconnectedness of these processes in ATP production.Figure 3. Diagram of Oxidative Phosphorylation: This diagram provides a comprehensive overview of oxidative phosphorylation, showing the citric acid cycle providing NADH and FADH2 to the electron transport chain. It illustrates how the ETC pumps protons to create a gradient, which ATP synthase then utilizes to generate ATP. This diagram effectively summarizes the entire process of ATP generation through the electron transport chain and chemiosmosis.
The Crucial Role of Oxygen as the Final Electron Acceptor
Oxygen’s role as the final electron acceptor in the electron transport chain is paramount for aerobic life. Without oxygen to accept electrons at the end of the chain, the entire system would back up, redox reactions would cease, proton pumping would stop, and ATP synthesis would halt. The reduction of oxygen to water is not just a byproduct; it is an essential step that allows the electron transport chain to continue functioning. Diagram electron transport chain illustrations invariably highlight oxygen’s position at the end of the chain, accepting electrons and hydrogen ions to form water.
ATP Yield and Efficiency
While theoretically, each NADH molecule can lead to the production of approximately 2.5 ATP molecules and each FADH2 to about 1.5 ATP molecules, the actual ATP yield from glucose catabolism is somewhat variable and often slightly lower. Factors such as proton leakage across the membrane and the energy cost of transporting ATP out of the mitochondria can influence the final ATP yield. Furthermore, intermediate molecules from these pathways are often diverted for other biosynthetic processes within the cell. Despite these variations, the electron transport chain remains the most efficient ATP-generating process in aerobic respiration, extracting a significant portion of the energy stored in glucose.
In Summary: Visualizing the Electron Transport Chain
The diagram electron transport chain is an invaluable tool for understanding the complex process of oxidative phosphorylation. By visualizing the components – Complexes I-IV, CoQ, cytochrome c, and ATP synthase – and the flow of electrons and protons, we can grasp the mechanism by which the electron transport chain generates the majority of ATP in aerobic respiration. The chain of redox reactions, coupled with proton pumping and chemiosmosis, represents a remarkable example of energy transduction in living systems. Understanding the diagram electron transport chain is fundamental to comprehending cellular energy metabolism and its vital role in life.
