The Dopamine Transporter (DAT) is a critical protein in the brain, belonging to the superfamily of Na+/Cl− dependent neurotransmitter transporters. Its primary function is to regulate dopamine levels in the brain by actively removing dopamine from the synapse and transporting it back into neurons. This reuptake process is essential for controlling dopamine signaling, which plays a vital role in various brain functions and behaviors. Understanding the dopamine transporter is crucial as it is implicated in a range of neuropsychiatric and neurodegenerative disorders, as well as substance abuse. Dysregulation of DAT and consequently dopamine levels can contribute to the development or increased susceptibility to these conditions.
Normal variations exist in DAT protein levels among individuals, particularly influenced by age. However, significant deviations from this normal range are observed in pathological states. Notably, a marked reduction in dopamine transporter levels is found in Parkinson’s disease and Lesch-Nyhan syndrome. Conversely, elevated DAT levels have been associated with attention deficit hyperactivity disorder (ADHD) (Dougherty et al., 1999, Dresel et al., 2000, Cheon et al., 2003, Krause et al., 2003, Madras et al., 2002) and Tourette’s Syndrome (Malison et al., 1995, Cheon et al., 2004). Furthermore, chronic stimulant drug use, such as cocaine, leads to an increase in dopamine transporter levels during withdrawal. In contrast, amphetamine use can result in dopamine transporter depletion, potentially due to neurotoxicity or amphetamine-induced DAT internalization. These fluctuations highlight the dopamine transporter’s dynamic role and its sensitivity to various factors.
The Genetic Blueprint of the Human Dopamine Transporter
The human dopamine transporter gene provides the genetic code for this essential protein. Spanning over 64 kb, the gene comprises 15 exons that encode the DAT protein. While the protein-coding region remains consistent in length, the 3′-untranslated region (3′-UTR) exhibits length variability due to a polymorphic variable number tandem repeat (VNTR) region (Vandenbergh et al., 1992). This VNTR consists of repeating units of a 40-base sequence, ranging from 3 to over 11 copies.
Numerous studies have explored the potential link between specific dopamine transporter gene alleles, defined by the VNTR repeat number, and susceptibility to dopamine-related disorders. These disorders include Parkinson’s disease, schizophrenia, delusional disorder, challenges in smoking cessation, polysubstance abuse, and alcoholism. Among these investigations, a recurring finding is the association of a ten-copy allele with attention deficit hyperactivity disorder (ADHD). Although the precise mechanisms are still under investigation, this connection has focused ADHD research on the dopamine transporter. As a primary target for anti-hyperactivity medications, the dopamine transporter in the brain, and potentially its elevated levels in adults with ADHD, are key areas of study. High dopamine transporter levels may arise from disruptions in the regulation of DAT protein expression by the transporter gene itself.
These findings raise the critical question of whether the number of repeat sequences in the 3′-UTR of the dopamine transporter gene influences DAT protein levels in the brain. Research has investigated the relationship between dopamine transporter genotype and phenotype by measuring DAT levels in the living human brain striatum using single photon emission computed tomography (SPECT) and genotyping DAT alleles based on VNTR repeat numbers in the same individuals (Jacobsen et al., 2000; Heinz et al., 2000; Martinez et al., 2001). Interestingly, these studies reported conflicting results, with subjects carrying nine-repeat alleles showing either lower or higher dopamine transporter levels compared to those with ten-repeat alleles. This discrepancy suggests that allele diversity might exist independently of the DAT 3′-UTR length (Miller et al., 2001; Miller and Madras, 2002), highlighting the complexity of dopamine transporter genetics.
Exploring the Dopamine Transporter Gene in Rhesus Monkeys
While a spontaneously hypertensive rat strain exhibits hyperactivity and serves as an ADHD model, rodents lack analogous repeat sequences in the 3′-UTR of their dopamine transporter gene. This makes rodents unsuitable for studying the impact of DAT alleles with specific lengths on hyperactivity. Given the evolutionary closeness of rhesus monkeys to humans, researchers hypothesized that a similar repeat sequence might be present in the rhesus monkey dopamine transporter gene. This led to investigations into several key questions:
- Does the rhesus monkey dopamine transporter gene contain a tandem repeat sequence?
- If present, is this sequence associated with activity levels in rhesus monkeys?
- Are there other polymorphisms in the monkey DAT gene, and do they correlate with activity levels?
- Can these polymorphisms in both monkeys and humans influence dopamine transporter protein expression levels?
The aim was to determine if a tandem repeat sequence existed in the 3′-untranslated region of the dopamine transporter gene in rhesus monkeys, whether the number of repeat units varied among individual monkeys, and if there was a link between the 3′-UTR of the DAT gene and hyperactivity in these primates.
Functional and Behavioral Implications of Dopamine Transporter Gene Polymorphisms
Similar to humans, but unlike other species previously examined, a fixed number tandem repeat (FNTR) sequence was found in the 3′-UTR of the monkey dopamine transporter gene (Figure 2.3). In the absence of a well-established animal model for ADHD, researchers compared the five most active rhesus monkeys to the five most sedentary ones from a behaviorally characterized group of 22 animals (Miller et al., 2001). Contrary to the human gene, the FNTR (comprising 39 bases per repeat and 12 repeats) was present in both highly active and sedentary monkeys, suggesting that DAT transcript length is not associated with hyperactivity in this species. However, further sequence analysis revealed potential single nucleotide polymorphisms (SNPs), one of which affects a Bst1107I restriction site. Screening of the entire monkey cohort confirmed that all animals had repeat regions of the same length, and digestion with Bst1107I was sufficient to differentiate two distinct FNTR alleles. While Bst1107I genotype showed some suggestive correlation, it was not a definitive predictor of hyperactive behavior (Miller et al., 2001). These findings led to the hypothesis that SNPs might also exist within human dopamine transporter VNTR alleles. Supporting this, cloning a portion of a novel ten-repeat allele of the human DAT gene revealed a Dral restriction site-sensitive SNP.
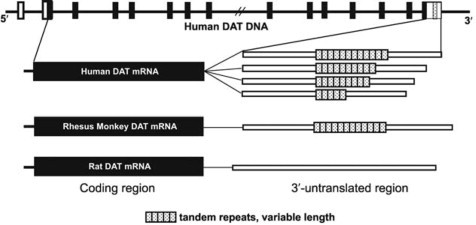 Schematic diagram comparing the dopamine transporter (DAT) gene in humans, rhesus monkeys, and rats, highlighting the variable number tandem repeat (VNTR) region in humans and fixed number tandem repeat (FNTR) in monkeys, relevant to dopamine regulation and related disorders like ADHD.
Schematic diagram comparing the dopamine transporter (DAT) gene in humans, rhesus monkeys, and rats, highlighting the variable number tandem repeat (VNTR) region in humans and fixed number tandem repeat (FNTR) in monkeys, relevant to dopamine regulation and related disorders like ADHD.
Figure 2.3. Schematic diagram depicting the human dopamine transporter (DAT) gene, implicated in ADHD, and a comparison of the human, rhesus monkey and rat DAT mRNAs. In the human DAT DNA, black boxes depict exons that make up the coding region, empty boxes depict non-coding exons, and the stippled box locates the position of a polymorphic variable number tandem repeat (VNTR) region within the portion of the gene that codes for the 3′-untranslated region. Human DAT mRNAs vary in length depending on how many repeated 40-base-pair sequences are present and each box represents one repeat sequence (e.g. 12 boxes = 12 repeats). Similar to human, the rhesus monkey DAT gene contains a series of tandem repeats in the 3′-untranslated region but, thus far, only a fixed number of tandem repeats (FNTR = 12 repeats) have been identified in >24 rhesus monkeys. The rat DAT gene does not contain analogous repeat sequences in the 3′-UTR.
Considering that different alleles of the dopamine transporter gene may contribute differently to altered dopamine transporter protein levels, it’s crucial to examine both VNTR length variations and SNPs as potential modulators. Using a reporter assay, researchers investigated if both the number of repeat sequences and specific SNPs in the 3′-UTR of human and rhesus monkey DAT genes could modify gene expression levels (Miller and Madras, 2002). In the human sequence, the number of tandem repeat sequences in the VNTR region of the DAT 3′-UTR significantly influenced reporter gene expression levels. Vectors with the nine-repeat sequence showed higher reporter gene expression than those with a ten-repeat sequence. SNPs also played a role, as human DAT 3′-UTR segments containing either an enzyme-sensitive or insensitive nucleotide (depending on the allele) resulted in notable differences in reporter gene expression levels. Intriguingly, the SNP effect was dependent on the vector promoter (Figure 2.4), demonstrating that the functional outcome of a particular SNP is context-dependent. This helps explain discrepancies in SNP association studies and emphasizes the need to define haplotypes. Although the frequency of this SNP in the human population is not fully known, it occurs in approximately 68% of fixed-length allele PCR products sensitive to Bst1107I digestion in rhesus monkeys. Despite differences in the specific DNA sequence, repeat number, and specific SNPs between rhesus monkey and human DAT 3′-UTR, the polymorphic structure, gene location, and, particularly, the functional effects of these polymorphisms on gene expression regulation showed striking parallels.
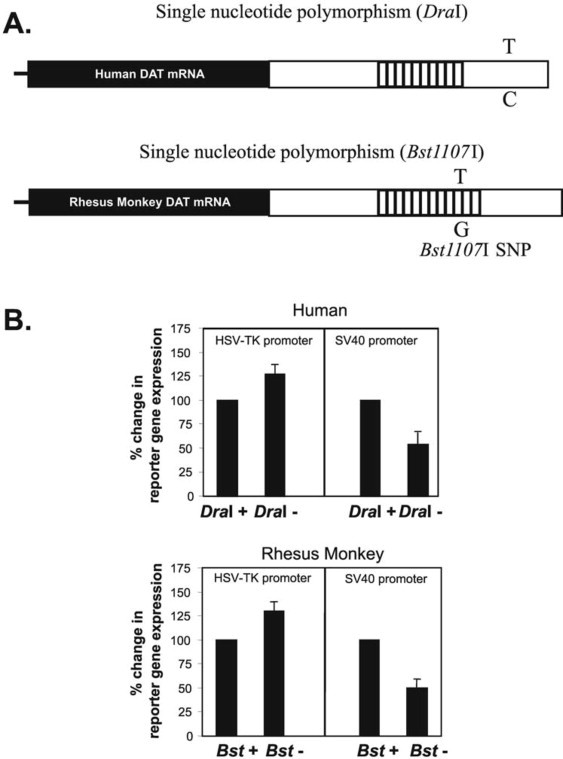 Graph illustrating the impact of single nucleotide polymorphisms (SNPs) in the 3'-untranslated region of the dopamine transporter (DAT) gene on gene expression levels in humans and rhesus monkeys, showing the functional consequences of genetic variations in DAT and their potential link to dopamine-related conditions.
Graph illustrating the impact of single nucleotide polymorphisms (SNPs) in the 3'-untranslated region of the dopamine transporter (DAT) gene on gene expression levels in humans and rhesus monkeys, showing the functional consequences of genetic variations in DAT and their potential link to dopamine-related conditions.
Figure 2.4. Effects of a single nucleotide polymorphism in the 3-untranslated region of the dopamine transporter gene on expression of reporter gene levels. A (top): Schematic comparison of the location of a single nucleotide polymorphism in a region of the DAT gene implicated in ADHD. Both the human DAT mRNA and rhesus monkey DAT mRNA have a tandem repeat region and single nucleotide polymorphisms. The coding region is shown in black and the 3′-untranslated region is shown in white. The location of the tandem repeat regions (open boxes) and the single nucleotide polymorphisms (T/C, human; T/G, monkey) that alter restriction endonuclease-sensitive sequences for Dral (human) and Bst1107| (rhesus monkey) are also shown. B (bottom): The effects of polymorphisms of human and rhesus monkey 3′-untranslated regions on luciferase reporter gene expression. Two (ten-repeat-containing) human DAT 3′-untranslated regions (Dral-sensitive and Dral-insensitive, top) are compared to two rhesus monkey 3′-untranslated regions (Bst1107I-sensitive vs. Bst1107|-insensitive, bottom) are shown. Although the polymorphism in human and rhesus monkey differed in sequence, the magnitude and direction of the promoter-dependent reporter protein levels were parallel. Data is adapted from Miller and Madras, 2002. Copyright © 2002
These studies yielded four significant findings: Firstly, a tandem repeat region, previously known in humans but not rodents, exists in the 3′-UTR of the rhesus monkey dopamine transporter gene. Secondly, while the repeat region length varies in humans, it is fixed in monkeys. Thirdly, the sequence of the repeat region in the monkey DAT gene varies between animals, and both human and monkey DAT genes contain SNPs in this region. Finally, the research linked genetic variations in the dopamine transporter gene to differences in gene expression and spontaneous activity levels in monkeys. Crucially, these data suggest that SNPs contribute to a diversity of dopamine transporter alleles beyond just VNTR region length, implying that specific sequence-defined haplotypes could differentially contribute to dopamine-related disorders.
Future Directions: Dopamine Transporter Polymorphisms and Brain Imaging
A key question is whether dopamine transporter gene polymorphisms correlate with dopamine transporter protein levels in the living brain. Imaging agents that non-invasively label the DAT have enabled the quantification of DAT density in vivo. As mentioned earlier, contradictory findings emerged when comparing individuals with nine- and/or ten-repeat length alleles (Jacobsen et al., 2000; Heinz et al., 2000; Martinez et al., 2001). Given the slight but significant association of ADHD with DAT ten-repeat length alleles, several studies investigated if dopamine transporter levels in ADHD brains deviate from the normal range. In three out of four SPECT studies on adults with ADHD, elevated dopamine transporter protein levels were observed, although genotyping was not performed in these cohorts (Dougherty et al., 1999; Krause et al., 2003; Dresel et al., 2000; van Dyck et al., 2002). Considering that ADHD is likely polygenic, and the ten-repeat length allele association accounts for a small variance in hyperactive-impulsive and inattentive symptoms (Waldman et al., 1998), a strong correlation between DAT (length) genotype and DAT density is unlikely. However, a detailed analysis of SNP frequency across human ten-repeat length-containing alleles is needed to further explore dopamine transporter gene polymorphisms and their links to ADHD and other dopamine-related disorders.
In conclusion, the relationship between the 3′-UTR sequence, dopamine transporter gene expression, and behavior remains to be fully elucidated. Other SNPs may also influence gene expression levels. The relevance of these findings to dopamine transporter gene regulation in vivo necessitates a thorough determination of SNP location and frequency in the human DAT 3′-UTR, as well as the 5′-UTR and other non-coding regions. The recent discovery of SNPs in the 5′-flanking region of the human dopamine transporter gene (Rubie et al., 2001) suggests that functional SNPs might exist in the promoter or other 5′ regulatory elements of the DAT gene. The current data implies that a dopamine transporter 3′-UTR with a specific sequence might function differently depending on the sequence of the DAT promoter or other DAT gene regions within a given haplotype. These findings underscore the importance of investigating the interaction between the native dopamine transporter promoter and 3′-UTR of relevant polymorphisms directly, or in appropriate cell lines that closely mimic dopamine neurons.
Similar to studies on mu-opioid polymorphisms, these dopamine transporter studies reveal parallels between rhesus monkeys and humans at the levels of genetic polymorphisms, their function, and phenotypic associations. Despite differences in specific DNA sequences, the conserved gene location, the functional effects of polymorphisms on gene expression, and the similar association of polymorphisms with behavior exhibit striking similarities, further emphasizing the translational relevance of primate models in understanding complex human conditions related to dopamine signaling.
