Cellular respiration is the fundamental process that fuels life, converting nutrients into usable energy in the form of ATP (adenosine triphosphate). While glycolysis and the citric acid cycle are crucial initial steps, the vast majority of ATP production during aerobic respiration hinges on the Electron Transport Chain Complex. This intricate system, also known as the respiratory chain, is a series of protein complexes embedded within the inner mitochondrial membrane of eukaryotic cells and the plasma membrane of prokaryotes. It’s the final pathway in glucose metabolism that harnesses atmospheric oxygen to generate a substantial amount of ATP, making it indispensable for aerobic life.
 This illustration shows the electron transport chain embedded in the inner mitochondrial membrane. The electron transport chain consists of four electron complexes. Complex I oxidizes NADH to NAD^^{+} and simultaneously pumps a proton across the membrane to the inter membrane space. The two electrons released from NADH are shuttled to coenzyme Q, then to complex III, to cytochrome c, to complex IV, then to molecular oxygen. In the process, two more protons are pumped across the membrane to the intermembrane space, and molecular oxygen is reduced to form water. Complex II removes two electrons from FADH_{2}, thereby forming FAD. The electrons are shuttled to coenzyme Q, then to complex III, cytochrome c, complex I, and molecular oxygen as in the case of NADH oxidation.
This illustration shows the electron transport chain embedded in the inner mitochondrial membrane. The electron transport chain consists of four electron complexes. Complex I oxidizes NADH to NAD^^{+} and simultaneously pumps a proton across the membrane to the inter membrane space. The two electrons released from NADH are shuttled to coenzyme Q, then to complex III, to cytochrome c, to complex IV, then to molecular oxygen. In the process, two more protons are pumped across the membrane to the intermembrane space, and molecular oxygen is reduced to form water. Complex II removes two electrons from FADH_{2}, thereby forming FAD. The electrons are shuttled to coenzyme Q, then to complex III, cytochrome c, complex I, and molecular oxygen as in the case of NADH oxidation.
Image depicting the electron transport chain within the mitochondrial membrane, showcasing the electron flow through complexes I-IV and proton pumping.
The electron transport chain (ETC) is a marvel of biological engineering, acting like a molecular assembly line for energy conversion. It’s a sequence of redox reactions, where electrons are passed from one component to the next in a carefully orchestrated manner. Think of it as a biological “bucket brigade,” efficiently transferring electrons until they ultimately reduce molecular oxygen (O2), the final electron acceptor, to form water (H2O). This process not only generates water but, more importantly, establishes a proton gradient that drives ATP synthesis.
The ETC is composed of four major protein complexes, labeled I through IV, along with mobile electron carriers. These complexes work in concert to extract energy from electron carriers NADH and FADH2, generated during earlier stages of cellular respiration like glycolysis and the citric acid cycle. The collective of these complexes and carriers is what we refer to as the electron transport chain complex. While eukaryotes house this machinery in the inner mitochondrial membrane, prokaryotes, lacking mitochondria, locate their ETC in the plasma membrane. Interestingly, some prokaryotic ETCs can function anaerobically, utilizing electron acceptors other than oxygen, highlighting the versatility of this system across different life forms. However, a consistent feature across all ETCs is their ability to pump protons, creating a crucial proton gradient across a membrane, which is the cornerstone of ATP production via chemiosmosis.
Complex I: NADH Dehydrogenase – The Entry Point for NADH Electrons
The electron transport chain begins with Complex I, also known as NADH dehydrogenase. This massive protein complex, one of the largest in the respiratory chain, is the gateway for electrons derived from NADH. NADH, produced in glycolysis and the citric acid cycle, carries high-energy electrons. Complex I accepts these electrons and initiates their journey down the ETC.
Complex I contains a crucial prosthetic group called flavin mononucleotide (FMN), a derivative of vitamin B2 (riboflavin). Prosthetic groups are non-protein molecules essential for protein function, and in this case, FMN plays a vital role in electron transfer. Complex I also incorporates iron-sulfur (Fe-S) clusters, inorganic cofactors that further facilitate electron movement. The enzyme NADH dehydrogenase within Complex I catalyzes the oxidation of NADH back to NAD+, releasing two electrons in the process.
Crucially, Complex I is not just an electron conduit; it’s also a proton pump. As electrons are transferred through Complex I, energy is released, which is harnessed to pump four hydrogen ions (protons, H+) across the inner mitochondrial membrane, from the mitochondrial matrix into the intermembrane space. This translocation of protons contributes directly to the establishment of the electrochemical gradient, the driving force behind ATP synthesis.
Coenzyme Q (Ubiquinone) and Complex II: FADH2’s Entry and Electron Shuttling
Complex II, also called succinate dehydrogenase, provides an alternative entry point for electrons into the electron transport chain. Unlike Complex I which accepts electrons from NADH, Complex II receives electrons from FADH2, another electron carrier generated in the citric acid cycle. FADH2 doesn’t interact with Complex I directly; instead, its electrons enter the ETC at Complex II.
A key mobile electron carrier, ubiquinone (Q), also known as coenzyme Q, bridges Complex I and Complex II to Complex III. Ubiquinone is a lipid-soluble molecule, granting it the freedom to move within the hydrophobic core of the inner mitochondrial membrane. It acts as an electron shuttle, collecting electrons from both Complex I (via NADH) and Complex II (via FADH2). Upon accepting electrons, ubiquinone becomes reduced to ubiquinol (QH2).
It’s important to note that electrons entering through Complex II, from FADH2, bypass the proton-pumping action of Complex I. This bypass has a consequence: fewer protons are pumped across the membrane when FADH2 is the electron donor compared to NADH. Since the proton gradient is directly linked to ATP production, FADH2 ultimately leads to the synthesis of fewer ATP molecules than NADH. The number of ATP molecules generated is directly proportional to the number of protons pumped across the inner mitochondrial membrane.
Complex III: Cytochrome bc1 Complex – Proton Pumping and Electron Transfer to Cytochrome c
Complex III, also known as cytochrome bc1 complex or ubiquinol-cytochrome c reductase, is the next major player in the electron transport chain. This complex receives electrons from reduced ubiquinone (QH2). Complex III is a multi-subunit protein containing cytochromes (cytochrome b and cytochrome c1), and an iron-sulfur protein (Rieske protein).
Cytochromes are proteins that contain heme prosthetic groups. Heme is a porphyrin ring complex with a central iron atom, similar to the heme found in hemoglobin responsible for oxygen transport in blood. However, in cytochromes, the heme iron is involved in electron transfer, not oxygen binding. The iron atom in heme can exist in two oxidation states, Fe2+ (reduced) and Fe3+ (oxidized), allowing it to accept and donate electrons as they move through the ETC.
Complex III performs two critical functions: it pumps protons across the inner mitochondrial membrane, further contributing to the proton gradient, and it facilitates the transfer of electrons from ubiquinol to cytochrome c, another mobile electron carrier. Cytochrome c is a peripheral membrane protein located in the intermembrane space. While ubiquinone carries electrons in pairs, cytochrome c can only accept and carry one electron at a time. Complex III effectively manages this transition, ensuring efficient electron flow.
Complex IV: Cytochrome c Oxidase – The Final Electron Acceptor and Water Formation
Complex IV, cytochrome c oxidase, is the terminal complex of the electron transport chain and plays the crucial role of catalyzing the final electron transfer to oxygen. This complex is composed of cytochrome a and cytochrome a3, along with copper ions. Cytochrome c oxidase receives electrons from cytochrome c.
Within Complex IV, a critical event occurs: molecular oxygen (O2) is reduced. The complex holds an oxygen molecule tightly between a heme iron in cytochrome a3 and a copper ion until it is fully reduced. This reduction of oxygen requires four electrons and four protons, ultimately resulting in the formation of two molecules of water (H2O). Water is the final byproduct of the electron transport chain.
Furthermore, Complex IV is also a proton pump, contributing to the proton gradient by pumping protons from the mitochondrial matrix to the intermembrane space during electron transfer. The removal of hydrogen ions from the mitochondrial matrix during water formation also indirectly contributes to the electrochemical gradient. Cytochrome c oxidase is essential for aerobic respiration; its inhibition, for example by cyanide, is rapidly fatal because it halts ATP production.
Chemiosmosis: Harnessing the Proton Gradient for ATP Synthesis
The electron transport chain, through the action of Complexes I, III, and IV, creates an electrochemical gradient across the inner mitochondrial membrane. This gradient is established by the uneven distribution of protons (H+), resulting in a higher concentration of protons in the intermembrane space compared to the mitochondrial matrix. This difference in proton concentration creates both a pH gradient (lower pH in the intermembrane space, higher pH in the matrix) and an electrical potential gradient (positive charge in the intermembrane space). This combined electrochemical gradient represents stored potential energy, much like water behind a dam.
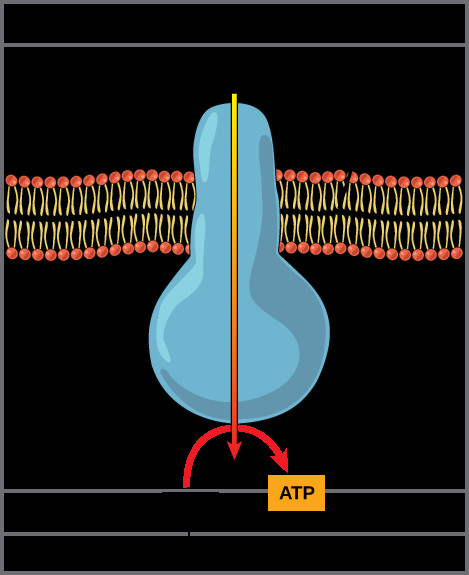 This illustration shows an ATP synthase enzyme embedded in the inner mitochondrial membrane. ATP synthase allows protons to move from an area of high concentration in the intermembrane space to an area of low concentration in the mitochondrial matrix. The energy derived from this exergonic process is used to synthesize ATP from ADP and inorganic phosphate.
This illustration shows an ATP synthase enzyme embedded in the inner mitochondrial membrane. ATP synthase allows protons to move from an area of high concentration in the intermembrane space to an area of low concentration in the mitochondrial matrix. The energy derived from this exergonic process is used to synthesize ATP from ADP and inorganic phosphate.
Image depicting ATP synthase utilizing the proton gradient to synthesize ATP.
Chemiosmosis is the process by which this stored energy in the proton gradient is used to drive ATP synthesis. The inner mitochondrial membrane is impermeable to protons, except through a specific channel provided by ATP synthase, also known as Complex V. ATP synthase is a remarkable molecular machine that spans the inner mitochondrial membrane.
As protons flow down their electrochemical gradient, from the intermembrane space back into the mitochondrial matrix through ATP synthase, the energy released by this exergonic movement is harnessed by ATP synthase. This flow of protons causes a physical rotation of a part of ATP synthase. This mechanical rotation drives the catalytic site of ATP synthase to phosphorylate ADP (adenosine diphosphate) by adding inorganic phosphate (Pi), thereby generating ATP. This process of ATP synthesis, driven by the proton gradient generated by the electron transport chain, is called oxidative phosphorylation, accounting for the vast majority of ATP produced during aerobic respiration.
ATP Yield and Efficiency
The theoretical maximum ATP yield from the complete oxidation of one glucose molecule through cellular respiration is often estimated to be around 36-38 ATP molecules in eukaryotes. However, the actual ATP yield in living cells is often lower and can vary. Several factors contribute to this variability.
Firstly, the efficiency of proton pumping by the ETC complexes can vary slightly between species and even within different tissues of the same organism. The number of protons pumped per NADH or FADH2 molecule can influence the final ATP yield.
Secondly, the shuttle systems used to transport cytosolic NADH (generated during glycolysis in the cytoplasm) into the mitochondria can affect ATP production. NADH itself cannot directly cross the inner mitochondrial membrane. Electrons from cytosolic NADH are transferred into the mitochondrial matrix using shuttle systems, such as the malate-aspartate shuttle and the glycerol-3-phosphate shuttle. The malate-aspartate shuttle, more prevalent in liver, kidney, and heart, effectively transfers electrons to mitochondrial NADH, preserving the potential to generate approximately 2.5 ATP per cytosolic NADH. However, the glycerol-3-phosphate shuttle, more active in muscle and brain, transfers electrons to FAD, leading to the production of only about 1.5 ATP per cytosolic NADH. The predominant shuttle system in a given tissue influences the overall ATP yield.
Thirdly, the proton gradient generated by the ETC is not exclusively used for ATP synthesis. It is also utilized to drive other mitochondrial processes, such as the transport of molecules like pyruvate and phosphate into the mitochondrial matrix. This “proton leak” reduces the number of protons available for ATP synthesis, slightly lowering the actual ATP yield.
Despite these variations and inefficiencies, the electron transport chain and oxidative phosphorylation remain remarkably efficient energy conversion systems. Overall, cellular respiration captures approximately 34% of the energy stored in a glucose molecule and converts it into ATP, the readily usable energy currency of the cell. The remaining energy is released as heat, contributing to body temperature in warm-blooded animals.
In Summary: The Electron Transport Chain Complex – A Symphony of Energy Conversion
The electron transport chain complex stands as a central pillar of aerobic life, orchestrating the final and most productive stage of cellular respiration. Through a series of precisely controlled redox reactions within Complexes I-IV, electrons from NADH and FADH2 are passed along the chain, ultimately reducing oxygen to water. Concurrently, this electron flow drives proton pumping across the inner mitochondrial membrane, establishing an electrochemical gradient. This gradient is then skillfully harnessed by ATP synthase to generate ATP through chemiosmosis.
The electron transport chain complex is not merely a linear pathway but a highly integrated system where each component plays a critical role in efficient energy transduction. From the initial electron entry at Complex I and Complex II, through the mobile carriers ubiquinone and cytochrome c, to the final reduction of oxygen at Complex IV, every step is essential for the generation of the proton gradient and subsequent ATP synthesis. Understanding the intricacies of the electron transport chain complex is fundamental to grasping the bioenergetics of life and the processes that sustain cellular activity.

