The Electron Transport Chain Cycle is a crucial sequence of protein complexes that plays a vital role in cellular energy production. This intricate system utilizes electrons derived from electron carriers to establish a chemical gradient, which is then harnessed to power oxidative phosphorylation, the process responsible for generating the majority of ATP in aerobic organisms. The chain itself is composed of a variety of molecules, including enzyme complexes, proteins, and peptides, working in concert to facilitate this energy conversion.
Oxidative phosphorylation, driven by the electron transport chain, is an exceptionally efficient method for producing large quantities of ATP, the fundamental energy currency of cellular metabolism. In this process, electrons are transferred from electron donors to electron acceptors through a series of reactions, ultimately leading to ATP synthesis. This phase stands out in cellular respiration as the primary site of ATP generation, far exceeding the ATP yield of other stages.
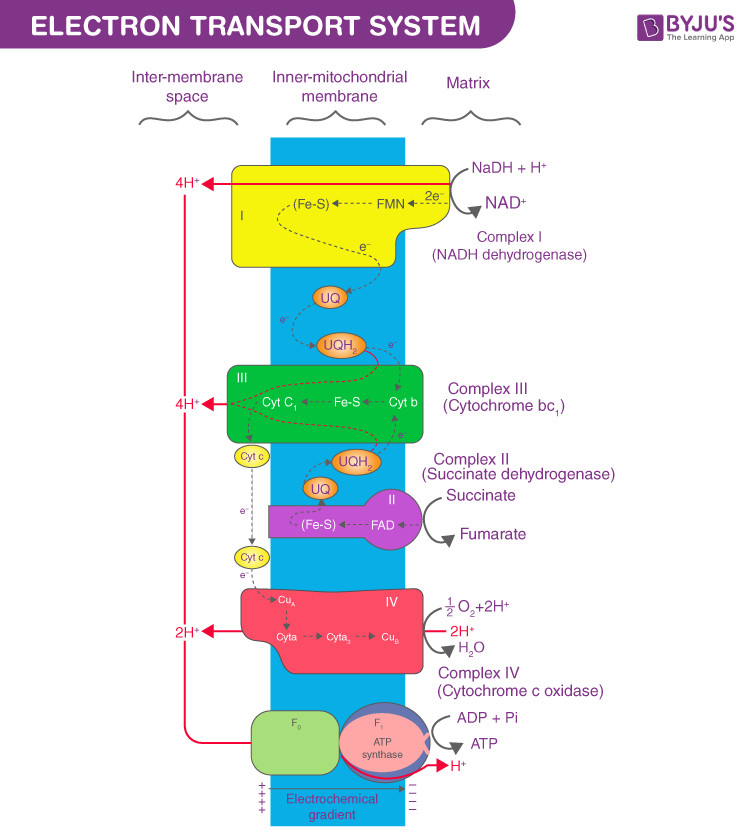 Diagram of the Electron Transport Chain in Mitochondria
Diagram of the Electron Transport Chain in Mitochondria
Electron transport can be visualized as a series of redox reactions, akin to a relay race where electrons are passed from one carrier to the next. This process is integral to aerobic respiration, distinguishing it as the only stage in glucose metabolism that directly utilizes atmospheric oxygen. As electrons progress along the chain, they eventually reduce molecular oxygen, resulting in the formation of water. This oxygen requirement in the final step underscores the essential role of oxygen in the overall chemical reaction of aerobic respiration, where both oxygen and glucose are consumed.
The Electron Transport Chain in Mitochondria: A Step-by-Step Breakdown
Within cells, the electron transport chain is primarily located in the inner mitochondrial membrane of eukaryotes and the plasma membrane of prokaryotes. This chain is organized into several protein complexes, each playing a distinct role in the electron transfer process.
Complex I: NADH-Q Oxidoreductase
Complex I, also known as NADH dehydrogenase, is the entry point for electrons derived from NADH. This complex contains enzymes incorporating flavin mononucleotide (FMN), a derivative of vitamin B2, and iron-sulfur clusters. Complex I accepts two electrons from NADH and transfers them to ubiquinone (Q), the mobile electron carrier.
Complex II: Succinate-Q Reductase
Complex II, or succinate dehydrogenase, serves as an alternative entry point for electrons, specifically from FADH2. FADH2, unlike NADH, does not pass electrons through Complex I; instead, it delivers them directly to Complex II. Similar to Complex I, Complex II also transfers electrons to ubiquinone (Q), effectively merging the electron flow from both NADH and FADH2.
Ubiquinone (Q): The Mobile Electron Carrier
Ubiquinone (Q), also known as coenzyme Q, is a small, lipid-soluble molecule that is freely mobile within the hydrophobic core of the inner mitochondrial membrane. Q acts as a crucial link between Complexes I and II and Complex III. It accepts electrons from Complexes I and II and diffuses through the membrane to deliver them to Complex III.
Complex III: Cytochrome c Reductase
Complex III, also called cytochrome bc1 complex, is responsible for further electron transfer and proton pumping. This complex is composed of cytochrome b, cytochrome c proteins, and iron-sulfur proteins. Complex III receives electrons from ubiquinone (Q) and passes them to cytochrome c, another mobile electron carrier. Importantly, Complex III also actively pumps protons from the mitochondrial matrix to the intermembrane space, contributing to the proton gradient.
Cytochrome c: The Intermembrane Shuttle
Cytochrome c is a small, water-soluble protein located in the intermembrane space of mitochondria. It acts as a mobile electron carrier, shuttling electrons between Complex III and Complex IV. Cytochrome c diffuses along the outer surface of the inner mitochondrial membrane, efficiently transferring electrons from Complex III to the next complex in the chain.
Complex IV: Cytochrome c Oxidase
Complex IV, or cytochrome c oxidase, is the final protein complex in the electron transport chain. It contains cytochromes a and a3, as well as copper centers. Complex IV receives electrons from cytochrome c and catalyzes the final redox reaction: the reduction of molecular oxygen to water. In this process, oxygen molecules bind to Complex IV and are progressively reduced, picking up hydrogen ions from the mitochondrial matrix to form water. This step is critical for maintaining the flow of electrons and regenerating the oxidized form of cytochrome c.
The Proton Gradient and ATP Synthesis
The pumping of protons by Complexes I, III, and IV across the inner mitochondrial membrane creates an electrochemical gradient, also known as the proton-motive force. This gradient represents a form of stored energy, with a higher concentration of protons in the intermembrane space compared to the mitochondrial matrix. The potential energy stored in this gradient is then harnessed by ATP synthase, an enzyme complex that allows protons to flow back down their concentration gradient into the matrix. This controlled flow of protons drives the rotation of ATP synthase, which in turn catalyzes the synthesis of ATP from ADP and inorganic phosphate. This process of ATP generation, powered by the electron transport chain and the proton gradient, is oxidative phosphorylation, the major source of cellular ATP in aerobic organisms.
In conclusion, the electron transport chain cycle is a remarkably efficient and elegantly organized system for energy conversion. Through a series of precisely orchestrated redox reactions and proton pumping, it generates the proton gradient necessary to drive ATP synthesis, providing the energy that fuels life processes in aerobic organisms.
