The cell membrane, or plasma membrane, acts as a selective barrier, meticulously controlling the passage of molecules and ions into and out of the cell. This dynamic interface, composed of a lipid bilayer, ensures that the cell receives essential nutrients and eliminates waste products, maintaining the internal environment necessary for life. While the lipid bilayer is permeable to water and certain small, uncharged molecules, the transport of most vital substances relies on specialized mechanisms, notably Facilitated Diffusion And Active Transport. These two processes are fundamental to cellular physiology, enabling cells to thrive in diverse environments. Understanding the nuances of facilitated diffusion and active transport is crucial to grasping cellular function at a fundamental level.
 Phospholipid bilayer of a cell membrane
Phospholipid bilayer of a cell membrane
Facilitated diffusion, a type of passive transport, empowers specific molecules and ions to cross the cell membrane down their concentration gradient with the assistance of membrane proteins. This process does not require the cell to expend metabolic energy, as it harnesses the inherent kinetic energy of molecules moving from an area of high concentration to an area of low concentration. Think of it as a slide allowing for an easier descent down a hill, rather than directly climbing over it. Facilitated diffusion is essential for the efficient uptake of many nutrients and the removal of waste products, ensuring that cellular processes are supplied with the necessary building blocks and cleared of potentially toxic byproducts.
Facilitated diffusion of ions is primarily mediated by ion channels, transmembrane proteins that create water-filled pores across the hydrophobic lipid bilayer. These channels are remarkably selective, often allowing only specific types of ions to pass through. Imagine them as gated tunnels, each designed for a particular type of passenger. Many ion channels are “gated,” meaning they can open and close in response to specific stimuli, allowing cells to regulate ion permeability dynamically. These gates can be triggered by various signals, including ligands, mechanical forces, voltage changes, and even light.
Ligand-gated ion channels respond to the binding of signaling molecules called ligands. These ligands can be extracellular, such as neurotransmitters, or intracellular, like second messengers. For instance, acetylcholine (ACh), a neurotransmitter, binds to receptors at synapses, opening channels that permit sodium ions (Na+) to flow into the cell, initiating nerve impulses or muscle contractions. Conversely, gamma-aminobutyric acid (GABA), another neurotransmitter, opens chloride ion (Cl-) channels in the central nervous system, inhibiting nerve impulse generation. Intracellular ligands, like cyclic AMP (cAMP) and cyclic GMP (cGMP), regulate ion channels involved in sensory responses like smell and sight. ATP, while primarily known as an energy molecule, also acts as an intracellular ligand, opening chloride and bicarbonate ion channels, although this process, despite ATP involvement in channel opening, is still considered facilitated diffusion as the ions move down their concentration gradient.
Mechanically-gated ion channels open in response to physical forces, such as pressure or stretch. Sound waves, for example, bend hair-like projections in the inner ear, triggering the opening of ion channels and generating nerve impulses that the brain interprets as sound. Similarly, stretch receptors in various tissues rely on mechanically-gated channels to detect changes in pressure or volume.
Voltage-gated ion channels are crucial in excitable cells like neurons and muscle cells. These channels open or close depending on changes in the electrical potential across the plasma membrane. During a nerve impulse, a change in voltage opens sodium channels in adjacent membrane regions, allowing a rapid influx of Na+ ions and propagating the nerve signal. The patch clamp technique, a Nobel Prize-winning method, has been instrumental in studying the properties of individual ion channels, revealing that they operate in an “all-or-none” fashion, either fully open or fully closed.
 Patch clamp technique setup
Patch clamp technique setup
Facilitated diffusion also plays a vital role in the transport of small, hydrophilic organic molecules like sugars and amino acids. Similar to ion transport, this process relies on transmembrane proteins. Some of these proteins form water-filled channels, while others may function as carrier proteins that undergo conformational changes upon binding to the transported molecule. Maltoporin, found in the outer membrane of E. coli, is an example of a channel protein that facilitates the diffusion of maltose and related disaccharides. In human red blood cells, glucose transporters (GLUTs) are carrier proteins that enable glucose to move from the blood into the cells. These transporters exhibit selectivity, ensuring that only specific molecules are transported. The interaction between the transported molecule and its transporter shares similarities with the enzyme-substrate relationship, highlighting the specificity and efficiency of facilitated diffusion.
Active transport, in contrast to facilitated diffusion, is an energy-requiring process that moves molecules or ions across the cell membrane against their concentration gradient. This “uphill” movement necessitates the input of energy, typically in the form of ATP. Active transport is essential for maintaining cellular homeostasis, allowing cells to concentrate specific substances inside or outside the cell, regardless of the natural direction of diffusion. This process relies on transmembrane proteins known as transporters or pumps.
Direct active transport, also known as primary active transport, directly utilizes the energy from ATP hydrolysis. These transporters bind ATP and use the energy released during its conversion to ADP to pump molecules against their concentration gradient.
The sodium-potassium pump (Na+/K+ ATPase) is a prime example of direct active transport and a critical protein in animal cells. This pump maintains the electrochemical gradients of sodium and potassium ions across the plasma membrane by expelling three sodium ions (Na+) out of the cell and importing two potassium ions (K+) into the cell for each ATP molecule hydrolyzed. These gradients are vital for several cellular functions, including establishing the resting membrane potential in nerve and muscle cells, maintaining osmotic balance to prevent cell swelling, and providing the driving force for indirect active transport mechanisms. The Na+/K+ ATPase consumes a significant portion of cellular ATP, highlighting its fundamental importance.
The hydrogen-potassium pump (H+/K+ ATPase), found in parietal cells of the stomach, is responsible for secreting gastric acid. This pump actively transports protons (H+) from the cytoplasm into the stomach lumen, creating a highly acidic environment essential for digestion. The calcium pump (Ca2+ ATPase) maintains a low cytosolic calcium concentration by pumping calcium ions (Ca2+) out of the cell or into intracellular storage compartments like the sarcoplasmic reticulum in muscle cells. This pump is crucial for regulating calcium-mediated signaling events, including muscle contraction. These P-type ion transporters, including Na+/K+ ATPase, H+/K+ ATPase, and Ca2+ ATPases, share a common mechanism involving protein phosphorylation and conformational changes during ion transport. Interestingly, these pumps can operate in reverse under certain conditions, synthesizing ATP when ions flow down their concentration gradient through the pump.
ABC transporters (ATP-Binding Cassette transporters) constitute a large family of transmembrane proteins that utilize ATP hydrolysis to transport a wide array of substrates across cell membranes. These transporters are characterized by their ATP-binding domains or “cassettes.” Examples of ABC transporters in humans include CFTR (cystic fibrosis transmembrane conductance regulator), mutations in which cause cystic fibrosis; TAP (transporter associated with antigen processing), involved in immune responses; transporters that export bile salts from liver cells; and multidrug resistance proteins that pump chemotherapeutic drugs out of cancer cells. The widespread presence of ABC transporters across different life forms underscores their evolutionary significance and diverse roles in cellular physiology.
Indirect active transport, or secondary active transport, harnesses the energy stored in the electrochemical gradient of one ion, typically sodium (Na+), established by direct active transport, to drive the transport of another molecule or ion against its concentration gradient. This process does not directly use ATP but relies on the potential energy created by primary active transport.
Symport pumps mediate indirect active transport where the driving ion and the transported molecule move in the same direction across the membrane. The sodium-glucose cotransporter (SGLT) is a key example of a symport pump. It utilizes the inward flow of Na+ ions down their electrochemical gradient to transport glucose into the cell against its concentration gradient. This transporter is crucial for glucose reabsorption in the kidneys and glucose uptake in the intestines. Similarly, symport pumps facilitate the uptake of amino acids, neurotransmitters, and iodide ions, leveraging the sodium gradient to drive their transport.
Antiport pumps, on the other hand, mediate indirect active transport where the driving ion and the transported molecule move in opposite directions across the membrane. The sodium-calcium exchanger (NCX) is an example of an antiport pump that uses the inward flow of Na+ ions to drive the outward transport of calcium ions (Ca2+), contributing to the maintenance of low intracellular calcium levels. In plant cells, sodium-proton antiporters in vacuoles utilize the proton gradient to sequester sodium ions, enabling plants to tolerate saline environments.
Disruptions in ion channel function due to inherited mutations can lead to various diseases, highlighting the critical role of facilitated diffusion in maintaining health. Cystic fibrosis, for example, is caused by mutations in a chloride channel, while certain forms of kidney stones are linked to defects in other chloride channels. Potassium channelopathies are associated with long QT syndrome, epilepsy in newborns, and some types of deafness. Sodium channel mutations can cause muscle spasms and Liddle’s syndrome, a form of hypertension. These inherited ion channel diseases underscore the delicate balance maintained by facilitated diffusion and the profound consequences of its disruption.
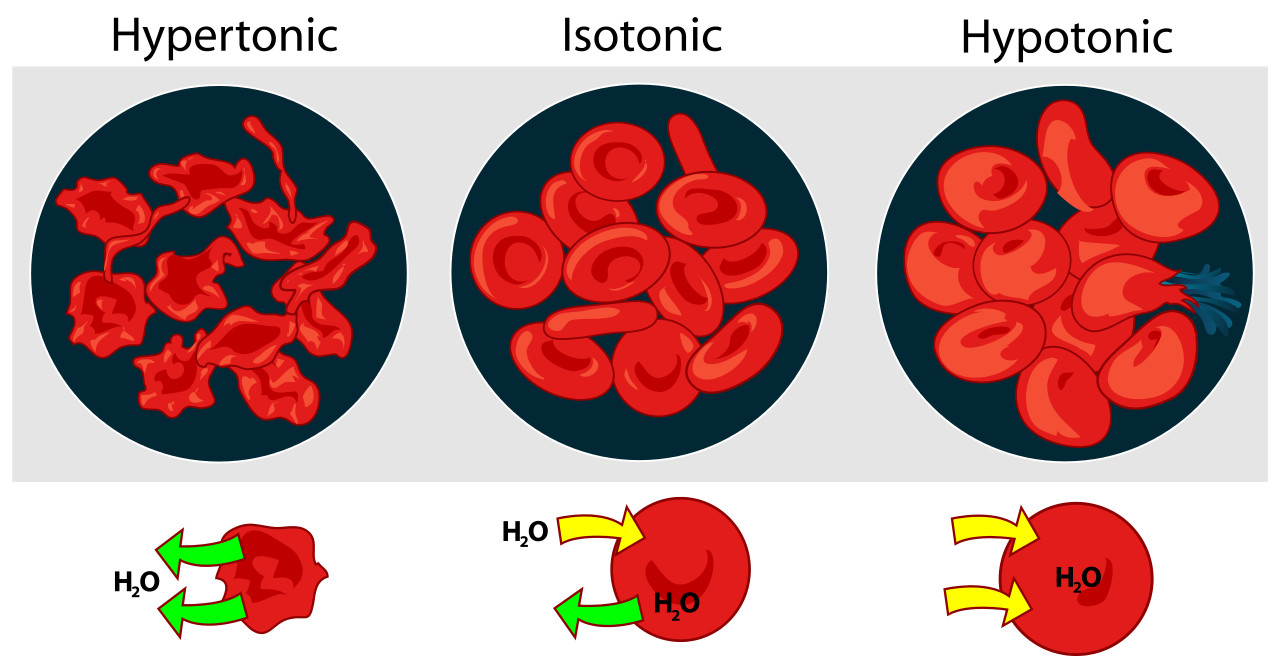
Osmosis, a specific type of facilitated diffusion, refers to the movement of water across a semipermeable membrane from an area of high water concentration to an area of low water concentration. While water can diffuse across the lipid bilayer to some extent, aquaporins, channel proteins specifically designed for water transport, greatly enhance this process. Osmosis is crucial for maintaining cell volume and osmotic balance. Cells respond differently to solutions with varying solute concentrations. In hypotonic solutions, where the external environment has a lower solute concentration than the cell, water rushes into the cell, potentially causing it to burst (hemolysis in red blood cells). Isotonic solutions have equal solute concentrations to the cell, resulting in no net water movement. Hypertonic solutions, with higher external solute concentrations, cause water to move out of the cell, leading to cell shrinkage (plasmolysis in plant cells and crenation in animal cells). Active transport of solutes plays a key role in regulating water movement and maintaining osmotic balance, demonstrating the interconnectedness of these transport mechanisms.
 Osmosis in red blood cells
Osmosis in red blood cells
In conclusion, both facilitated diffusion and active transport are indispensable processes for cell survival, ensuring the controlled movement of molecules and ions across the cell membrane. Facilitated diffusion, a passive process, utilizes channel and carrier proteins to expedite transport down concentration gradients, while active transport, an energy-dependent process, employs pumps to move substances against their concentration gradients. These two mechanisms, working in concert, maintain cellular homeostasis, enable nutrient uptake, waste removal, signal transduction, and a myriad of other essential cellular functions, underpinning the very foundation of life.