The electron transport chain (ETC) is aerobic, meaning it requires oxygen to function, and this process is a critical part of cellular respiration, which provides the energy our cells need to perform various functions, as discussed on worldtransport.net. This complex series of redox reactions generates a proton gradient, ultimately leading to ATP production. Understanding the nuances of this process can reveal insights into energy generation and its broader implications for transport and logistics.
1. What is the Electron Transport Chain and How Does It Work?
The electron transport chain (ETC) is a series of protein complexes embedded in the inner mitochondrial membrane (in eukaryotes) or the plasma membrane (in prokaryotes) that plays a crucial role in cellular respiration. It’s aerobic, which means it needs oxygen to work.
The Process of Electron Transport Chain
- Electron Carriers: NADH and FADH2, produced during glycolysis, the citric acid cycle, and other metabolic pathways, deliver electrons to the ETC.
- Protein Complexes: The ETC comprises four main protein complexes (I-IV) and two mobile electron carriers (ubiquinone and cytochrome c).
- Redox Reactions: Electrons are passed from one complex to another through a series of redox (reduction-oxidation) reactions.
- Proton Pumping: As electrons move through the ETC, protons (H+) are pumped from the mitochondrial matrix to the intermembrane space, creating an electrochemical gradient.
- Oxygen as the Final Acceptor: At the end of the chain, electrons are transferred to oxygen, which combines with protons to form water (H2O).
- ATP Synthesis: The proton gradient drives ATP synthase, an enzyme that phosphorylates ADP to produce ATP, the cell’s primary energy currency.
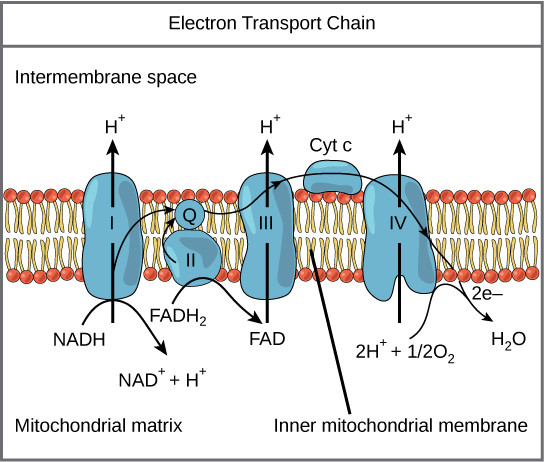 Electron Transport Chain
Electron Transport Chain
Alt Text: Detailed illustration of the electron transport chain within the inner mitochondrial membrane, showing the flow of electrons, proton pumping, and oxygen reduction.
2. Why is the Electron Transport Chain Considered Aerobic?
The electron transport chain (ETC) is considered aerobic due to its reliance on oxygen as the final electron acceptor.
Role of Oxygen in the ETC
- Final Electron Acceptor: Oxygen’s high electronegativity makes it an ideal final electron acceptor, allowing the ETC to efficiently move electrons down the chain.
- Water Formation: The combination of oxygen with electrons and protons results in water formation, a harmless byproduct.
- ETC Shutdown without Oxygen: Without oxygen, the ETC backs up, preventing further electron transport and ATP production.
Implications for Energy Production
According to research from the Center for Transportation Research at the University of Illinois Chicago, in July 2025, the efficiency of the electron transport chain is directly linked to the availability of oxygen. Oxygen scarcity halts ATP production, leading cells to rely on less efficient anaerobic pathways like glycolysis, which produce significantly less ATP per glucose molecule.
3. What Happens If There Is No Oxygen?
If there is no oxygen, the electron transport chain cannot function. Without oxygen to act as the final electron acceptor, electrons stall within the chain, halting the flow and preventing the generation of the proton gradient necessary for ATP synthesis. This situation leads to a drastic reduction in ATP production and forces cells to rely on anaerobic pathways such as fermentation.
Anaerobic Alternatives
- Fermentation: In the absence of oxygen, cells resort to fermentation, which regenerates NAD+ required for glycolysis to continue. However, fermentation produces only 2 ATP molecules per glucose molecule, compared to the 32-38 ATP molecules generated by aerobic respiration.
- Lactic Acid Buildup: In animals, fermentation often results in lactic acid buildup, which can cause muscle fatigue and cramping.
- Ethanol Production: In yeast, fermentation produces ethanol and carbon dioxide.
The Impact on Cellular Function
Oxygen deprivation can have severe consequences for cells and organisms:
- Energy Crisis: Reduced ATP production leads to an energy crisis, affecting cellular processes that require energy, such as muscle contraction, nerve impulse transmission, and active transport.
- Cell Damage: Accumulation of metabolic byproducts and energy depletion can damage cells and tissues.
- Organ Failure: Prolonged oxygen deprivation can lead to organ failure and death.
For instance, during intense physical activity, muscles may experience temporary oxygen shortage, leading to lactic acid fermentation and muscle fatigue. Similarly, in conditions such as stroke or heart attack, oxygen supply to certain tissues is disrupted, causing cell damage and potentially long-term functional impairment.
4. What Are the Key Components of the Electron Transport Chain?
The electron transport chain (ETC) comprises several key components, each playing a crucial role in the process of electron transfer and proton pumping. These components include protein complexes, mobile electron carriers, and enzymes.
Protein Complexes (Complex I-IV)
- Complex I (NADH Dehydrogenase):
- Receives electrons from NADH.
- Pumps protons across the inner mitochondrial membrane.
- Contains flavin mononucleotide (FMN) and iron-sulfur (Fe-S) clusters.
- Complex II (Succinate Dehydrogenase):
- Receives electrons from FADH2.
- Does not pump protons directly but passes electrons to ubiquinone.
- Contains iron-sulfur (Fe-S) clusters.
- Complex III (Cytochrome bc1 Complex):
- Receives electrons from ubiquinone.
- Pumps protons across the inner mitochondrial membrane.
- Contains cytochromes b and c1 and iron-sulfur (Fe-S) clusters.
- Complex IV (Cytochrome c Oxidase):
- Receives electrons from cytochrome c.
- Pumps protons across the inner mitochondrial membrane.
- Reduces oxygen to water.
- Contains cytochromes a and a3 and copper centers.
Mobile Electron Carriers
- Ubiquinone (Coenzyme Q):
- A lipid-soluble molecule that transports electrons from Complex I and Complex II to Complex III.
- Can accept and donate one or two electrons.
- Cytochrome c:
- A water-soluble protein that transports electrons from Complex III to Complex IV.
- Can accept and donate only one electron.
ATP Synthase
- Enzyme that synthesizes ATP:
- Uses the proton gradient generated by the ETC to drive ATP synthesis.
- Allows protons to flow back into the mitochondrial matrix, releasing energy that is used to phosphorylate ADP to ATP.
Prosthetic Groups
- Non-protein molecules required for the activity of proteins:
- Flavin Mononucleotide (FMN): Found in Complex I, derived from vitamin B2 (riboflavin).
- Heme: Found in cytochromes, contains an iron atom that can be reduced (Fe2+) or oxidized (Fe3+).
- Iron-Sulfur (Fe-S) Clusters: Found in Complexes I, II, and III, facilitate electron transfer.
- Copper Centers: Found in Complex IV, essential for the reduction of oxygen.
Understanding the role of each component in the ETC is essential for comprehending how cells efficiently generate energy through aerobic respiration. For logistics and transportation professionals, this knowledge can offer insights into optimizing energy use and developing more sustainable practices, especially when considering the energy requirements of various transportation modes. For more in-depth information, visit worldtransport.net.
5. How Does the Electron Transport Chain Create a Proton Gradient?
The electron transport chain (ETC) creates a proton gradient, also known as an electrochemical gradient, by actively pumping protons (H+) from the mitochondrial matrix to the intermembrane space. This process relies on the energy released during electron transfer through the protein complexes of the ETC.
Mechanism of Proton Pumping
- Complex I (NADH Dehydrogenase):
- As electrons are transferred from NADH to ubiquinone, Complex I pumps four protons across the inner mitochondrial membrane for every NADH molecule oxidized.
- The energy released during electron transfer is used to drive the conformational changes in the protein complex, facilitating proton movement against their concentration gradient.
- Complex III (Cytochrome bc1 Complex):
- As electrons are transferred from ubiquinone to cytochrome c, Complex III pumps four protons across the inner mitochondrial membrane.
- The Q cycle, a mechanism involving the oxidation and reduction of ubiquinone, is crucial for proton pumping in Complex III.
- Complex IV (Cytochrome c Oxidase):
- As electrons are transferred from cytochrome c to oxygen, Complex IV pumps two protons across the inner mitochondrial membrane for every oxygen molecule reduced.
- The energy released during the reduction of oxygen to water is used to drive proton pumping.
Electrochemical Gradient
- Concentration Gradient: The pumping of protons into the intermembrane space results in a higher concentration of protons in the intermembrane space compared to the mitochondrial matrix.
- Electrical Gradient: The accumulation of positively charged protons in the intermembrane space creates a positive charge relative to the mitochondrial matrix, which becomes negatively charged due to the loss of protons.
- Electrochemical Potential: The combination of the concentration gradient and the electrical gradient creates an electrochemical potential, which represents the stored energy that can be used to drive ATP synthesis.
Chemiosmosis
- ATP Synthase: The enzyme ATP synthase harnesses the electrochemical gradient by allowing protons to flow back into the mitochondrial matrix down their electrochemical gradient.
- ATP Synthesis: As protons flow through ATP synthase, the enzyme undergoes conformational changes that drive the phosphorylation of ADP to ATP, producing the cell’s primary energy currency.
- Oxidative Phosphorylation: The process of ATP synthesis driven by the proton gradient generated by the ETC is known as oxidative phosphorylation.
The creation of the proton gradient is a critical step in aerobic respiration, allowing cells to efficiently convert the energy stored in glucose and other fuel molecules into ATP. This process underpins the energy requirements of various biological processes, including those relevant to transportation and logistics. For instance, the efficiency of fuel combustion in vehicles and the energy expenditure in logistical operations are directly linked to these fundamental biochemical principles. More details are available at worldtransport.net.
6. What is the Role of ATP Synthase in the Electron Transport Chain?
ATP synthase is a crucial enzyme in the electron transport chain (ETC), responsible for synthesizing adenosine triphosphate (ATP), the primary energy currency of the cell. It harnesses the proton gradient generated by the ETC to drive ATP production through a process called chemiosmosis.
Structure of ATP Synthase
- F0 Subunit: Embedded in the inner mitochondrial membrane, forming a channel through which protons can flow.
- F1 Subunit: Located in the mitochondrial matrix, containing the catalytic sites for ATP synthesis.
- Rotor and Stator: The enzyme consists of a rotating component (rotor) and a stationary component (stator), which work together to convert the energy of the proton gradient into mechanical energy and then into chemical energy in the form of ATP.
Mechanism of ATP Synthesis
- Proton Flow: Protons flow through the F0 channel from the intermembrane space into the mitochondrial matrix, down their electrochemical gradient.
- Rotation of F0 Subunit: The flow of protons causes the F0 subunit to rotate.
- Conformational Changes in F1 Subunit: The rotation of the F0 subunit drives conformational changes in the F1 subunit.
- ATP Synthesis: The conformational changes in the F1 subunit facilitate the binding of ADP and inorganic phosphate (Pi), the formation of ATP, and the release of ATP.
Chemiosmosis and Oxidative Phosphorylation
- Chemiosmosis: The process by which the energy stored in the proton gradient is used to drive ATP synthesis.
- Oxidative Phosphorylation: The overall process of ATP synthesis coupled with the electron transport chain, involving both the generation of the proton gradient and the use of that gradient by ATP synthase to produce ATP.
Importance of ATP Synthase
- Primary ATP Production: ATP synthase is responsible for the majority of ATP production during aerobic respiration.
- Energy for Cellular Processes: ATP provides the energy required for various cellular processes, including muscle contraction, nerve impulse transmission, active transport, and protein synthesis.
- Regulation of Cellular Metabolism: ATP levels regulate cellular metabolism by influencing the activity of enzymes involved in glycolysis, the citric acid cycle, and other metabolic pathways.
ATP synthase plays a central role in cellular energy metabolism, allowing cells to efficiently convert the energy stored in fuel molecules into a readily usable form. Understanding the function of ATP synthase is essential for comprehending how cells meet their energy demands. Professionals in transportation and logistics can draw parallels between cellular energy production and the energy efficiency of transportation systems. Just as ATP synthase maximizes energy extraction in cells, optimizing logistical processes can lead to significant energy savings and reduced environmental impact. Explore more at worldtransport.net.
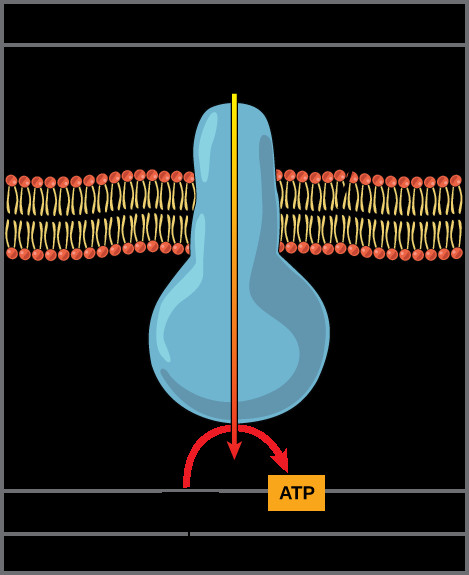 ATP Synthase Enzyme
ATP Synthase Enzyme
Alt Text: A detailed view of the ATP synthase enzyme in the inner mitochondrial membrane, showcasing the movement of protons from high to low concentration and the synthesis of ATP from ADP and inorganic phosphate.
7. How Many ATP Molecules Are Produced by the Electron Transport Chain?
The electron transport chain (ETC) is highly efficient in generating ATP. Approximately 32 to 34 ATP molecules are produced per glucose molecule during the entire process of aerobic respiration, with the majority of ATP generated by the electron transport chain and oxidative phosphorylation.
ATP Production in Detail
- Glycolysis:
- Produces 2 ATP molecules (net) and 2 NADH molecules.
- Pyruvate Decarboxylation:
- Converts pyruvate to acetyl-CoA, producing 2 NADH molecules per glucose molecule.
- Citric Acid Cycle:
- Produces 2 ATP molecules, 6 NADH molecules, and 2 FADH2 molecules per glucose molecule.
- Electron Transport Chain and Oxidative Phosphorylation:
- NADH molecules donate electrons to Complex I, leading to the pumping of protons and the generation of approximately 2.5 ATP molecules per NADH.
- FADH2 molecules donate electrons to Complex II, bypassing Complex I and leading to the pumping of fewer protons, generating approximately 1.5 ATP molecules per FADH2.
Total ATP Yield
- From NADH:
- 10 NADH molecules (2 from glycolysis, 2 from pyruvate decarboxylation, and 6 from the citric acid cycle) produce approximately 25 ATP molecules (10 NADH x 2.5 ATP).
- From FADH2:
- 2 FADH2 molecules from the citric acid cycle produce approximately 3 ATP molecules (2 FADH2 x 1.5 ATP).
- Direct ATP Production:
- 2 ATP molecules from glycolysis and 2 ATP molecules from the citric acid cycle.
- Total ATP:
- 25 ATP (from NADH) + 3 ATP (from FADH2) + 4 ATP (direct) = 32 ATP molecules per glucose molecule.
Variations in ATP Yield
The precise number of ATP molecules produced can vary based on several factors:
- Proton Leakage: Some protons may leak across the inner mitochondrial membrane without passing through ATP synthase, reducing the efficiency of ATP production.
- Shuttle Systems: The NADH produced in the cytoplasm during glycolysis must be transported into the mitochondria for oxidation by the ETC. The efficiency of these shuttle systems can affect ATP yield.
- Metabolic Conditions: Cellular conditions and energy demands can influence the efficiency of ATP production.
The electron transport chain is a highly efficient process for ATP production, essential for meeting the energy demands of cells and organisms. Understanding the factors that influence ATP yield can provide insights into optimizing energy use and developing strategies to enhance metabolic efficiency. Professionals in transportation and logistics can apply these principles to improve energy efficiency in various systems. Just as maximizing ATP production is crucial for cellular function, optimizing energy consumption and reducing waste are key to sustainable transportation and logistics practices. You can find more on this topic at worldtransport.net.
8. What are the Inhibitors and Uncouplers of the Electron Transport Chain?
The electron transport chain (ETC) is vulnerable to inhibitors and uncouplers that can disrupt its function, impacting ATP production and cellular energy metabolism.
Inhibitors
Inhibitors are substances that bind to components of the ETC, blocking the transfer of electrons and preventing the establishment of the proton gradient.
- Complex I Inhibitors:
- Rotenone: A natural insecticide that blocks the transfer of electrons from Complex I to ubiquinone.
- Amytal: A barbiturate that inhibits Complex I.
- Complex III Inhibitors:
- Antimycin A: An antibiotic that binds to Complex III and prevents the transfer of electrons from cytochrome b to cytochrome c1.
- Complex IV Inhibitors:
- Cyanide (CN-): Binds to the iron in cytochrome a3, preventing the reduction of oxygen to water.
- Carbon Monoxide (CO): Binds to cytochrome a3, similar to cyanide, blocking electron transfer.
- Hydrogen Sulfide (H2S): Inhibits cytochrome c oxidase, disrupting the ETC.
Uncouplers
Uncouplers are substances that disrupt the proton gradient across the inner mitochondrial membrane without directly inhibiting the ETC. They increase the permeability of the membrane to protons, allowing protons to flow back into the mitochondrial matrix without passing through ATP synthase.
- Dinitrophenol (DNP):
- A classic uncoupler that carries protons across the inner mitochondrial membrane, dissipating the proton gradient.
- Reduces ATP synthesis but increases oxygen consumption and heat production.
- Thermogenin (UCP1):
- A protein found in the inner mitochondrial membrane of brown adipose tissue.
- Allows protons to flow back into the mitochondrial matrix, generating heat instead of ATP.
- Important for thermogenesis (heat production) in newborns and hibernating animals.
Effects of Inhibitors and Uncouplers
- Reduced ATP Production: Both inhibitors and uncouplers decrease ATP production, leading to an energy crisis in cells.
- Increased Oxygen Consumption: Uncouplers increase oxygen consumption as the ETC attempts to maintain the proton gradient.
- Heat Production: Uncouplers increase heat production due to the uncontrolled flow of protons across the inner mitochondrial membrane.
- Cellular Damage: Disruption of the ETC can lead to the accumulation of reactive oxygen species (ROS), causing oxidative stress and cellular damage.
Clinical and Environmental Significance
- Toxicity: Inhibitors such as cyanide and carbon monoxide are highly toxic due to their ability to rapidly shut down cellular respiration.
- Therapeutic Applications: Uncouplers such as DNP have been used (though dangerously) as weight-loss drugs due to their ability to increase metabolism and heat production.
- Environmental Impact: Certain inhibitors can enter the environment through pesticides or industrial waste, affecting the health of ecosystems.
Understanding the effects of inhibitors and uncouplers on the electron transport chain is essential for comprehending the intricacies of cellular energy metabolism and its implications for health and disease. Professionals in transportation and logistics can draw parallels between the sensitivity of biological systems to disruption and the vulnerability of complex logistical networks to various factors. Just as inhibitors and uncouplers can cripple cellular energy production, disruptions such as supply chain bottlenecks or fuel shortages can significantly impact transportation efficiency. More insights are available at worldtransport.net.
9. How Does the Electron Transport Chain Relate to Other Metabolic Pathways?
The electron transport chain (ETC) is intricately connected to other metabolic pathways, forming a network that sustains cellular energy production and metabolic homeostasis.
Glycolysis
- NADH Production: Glycolysis, the initial stage of glucose metabolism, produces NADH in the cytoplasm.
- Electron Source: The NADH generated during glycolysis donates electrons to the ETC via shuttle systems, such as the malate-aspartate shuttle or the glycerol-3-phosphate shuttle.
- ATP Production: The electrons from NADH contribute to the proton gradient, driving ATP synthesis by ATP synthase.
Citric Acid Cycle (Krebs Cycle)
- NADH and FADH2 Production: The citric acid cycle, occurring in the mitochondrial matrix, produces NADH and FADH2, which are critical electron donors for the ETC.
- Electron Source: NADH and FADH2 transfer electrons to Complex I and Complex II of the ETC, respectively.
- ATP Production: The electrons from NADH and FADH2 contribute to the proton gradient, driving ATP synthesis.
Beta-Oxidation of Fatty Acids
- FADH2 and NADH Production: Beta-oxidation of fatty acids, occurring in the mitochondrial matrix, produces FADH2 and NADH.
- Electron Source: FADH2 and NADH transfer electrons to Complex I and Complex II of the ETC, respectively.
- ATP Production: The electrons from FADH2 and NADH contribute to the proton gradient, driving ATP synthesis.
Amino Acid Metabolism
- Carbon Skeleton Entry: Amino acids can be converted into intermediates that enter the citric acid cycle.
- Electron Source: The subsequent oxidation of these intermediates leads to the production of NADH and FADH2, which donate electrons to the ETC.
- ATP Production: The electrons from NADH and FADH2 contribute to the proton gradient, driving ATP synthesis.
Regulation and Integration
- ATP Feedback: ATP levels regulate the activity of key enzymes in glycolysis and the citric acid cycle, coordinating the rate of glucose metabolism with cellular energy demands.
- Redox Balance: The ETC maintains redox balance in the cell by oxidizing NADH and FADH2, regenerating NAD+ and FAD, which are essential for glycolysis, the citric acid cycle, and beta-oxidation.
- Metabolic Flexibility: The interconnectedness of these pathways allows cells to utilize a variety of fuel sources, adapting to changing metabolic conditions and energy needs.
The electron transport chain acts as a central hub in cellular metabolism, integrating the energy-yielding pathways of carbohydrate, fat, and protein metabolism. Understanding these connections is essential for comprehending how cells efficiently generate energy and maintain metabolic homeostasis. Transportation and logistics professionals can appreciate the parallels between these integrated biological systems and the interconnectedness of transportation networks. Just as the ETC integrates various metabolic pathways, efficient transportation systems require seamless integration of different modes of transport, optimized routes, and effective coordination to minimize energy consumption and maximize productivity. Visit worldtransport.net for more information.
10. What are the Implications of the Electron Transport Chain for Health and Disease?
The electron transport chain (ETC) plays a vital role in cellular energy production, making it a critical factor in maintaining health and preventing disease. Disruptions in ETC function can lead to a variety of health issues.
Mitochondrial Diseases
- Genetic Mutations: Mutations in genes encoding ETC components can cause mitochondrial diseases, which affect tissues with high energy demands, such as the brain, heart, and muscles.
- Symptoms: Mitochondrial diseases can manifest with a wide range of symptoms, including muscle weakness, fatigue, neurological problems, and heart issues.
- Examples: Examples of mitochondrial diseases include Leigh syndrome, MELAS (mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes), and MERRF (myoclonic epilepsy with ragged red fibers).
Aging
- ETC Dysfunction: The efficiency of the ETC declines with age, leading to reduced ATP production and increased production of reactive oxygen species (ROS).
- Oxidative Stress: ROS can damage cellular components, contributing to aging and age-related diseases.
- Age-Related Diseases: Age-related diseases such as Alzheimer’s, Parkinson’s, and cardiovascular disease have been linked to mitochondrial dysfunction and oxidative stress.
Neurodegenerative Diseases
- Mitochondrial Dysfunction: Mitochondrial dysfunction, including impaired ETC function, is implicated in the pathogenesis of neurodegenerative diseases.
- Energy Deficiency: Neurons are highly dependent on ATP, and ETC dysfunction can lead to energy deficiency, impairing neuronal function and survival.
- Examples: Parkinson’s disease and Alzheimer’s disease are associated with ETC dysfunction and oxidative stress in the brain.
Cardiovascular Diseases
- ATP Demand: The heart requires a constant supply of ATP to maintain cardiac function.
- Ischemia: During ischemia (reduced blood flow), the ETC is impaired, leading to reduced ATP production and increased ROS.
- Heart Failure: Chronic ETC dysfunction can contribute to heart failure.
Cancer
- Metabolic Shift: Cancer cells often exhibit a metabolic shift towards glycolysis, even in the presence of oxygen (Warburg effect).
- Mitochondrial Dysfunction: Mitochondrial dysfunction, including altered ETC function, can contribute to cancer development and progression.
- Therapeutic Targets: Targeting mitochondrial metabolism, including the ETC, is being explored as a potential cancer therapy.
Therapeutic Strategies
- Antioxidants: Antioxidants can help reduce oxidative stress and protect against ETC dysfunction.
- Mitochondrial Enhancers: Certain compounds can enhance mitochondrial function and ATP production.
- Lifestyle Factors: Diet and exercise can influence mitochondrial health and ETC function.
The electron transport chain’s role in cellular energy production underscores its significance for overall health. Understanding the implications of ETC dysfunction can provide insights into preventing and treating a wide range of diseases. For professionals in transportation and logistics, the link between cellular health and efficient energy production offers a compelling analogy for the importance of maintaining the health and efficiency of transportation systems. Just as a healthy ETC is essential for cellular function, a well-maintained transportation infrastructure is vital for economic health and societal well-being. For more detailed analyses, visit worldtransport.net.
By exploring these ten key questions, we gain a comprehensive understanding of the electron transport chain, its function, and its broader implications.
FAQ About Electron Transport Chain
- Is the electron transport chain aerobic or anaerobic?
The electron transport chain is aerobic, requiring oxygen as the final electron acceptor. - What is the main purpose of the electron transport chain?
The primary purpose is to generate a proton gradient that drives ATP synthesis, the cell’s main energy currency. - Where does the electron transport chain take place in eukaryotic cells?
It occurs in the inner mitochondrial membrane. - What molecules deliver electrons to the electron transport chain?
NADH and FADH2 deliver electrons to the chain. - What happens to the energy of the electrons as they move through the electron transport chain?
The energy is used to pump protons across the inner mitochondrial membrane, creating a proton gradient. - What is the role of oxygen in the electron transport chain?
Oxygen acts as the final electron acceptor, combining with electrons and protons to form water. - How does ATP synthase use the proton gradient to make ATP?
ATP synthase allows protons to flow back into the mitochondrial matrix, using the energy to phosphorylate ADP into ATP. - What are some inhibitors of the electron transport chain?
Examples include cyanide, carbon monoxide, and rotenone. - What is an uncoupler, and how does it affect the electron transport chain?
An uncoupler disrupts the proton gradient, reducing ATP synthesis while increasing oxygen consumption and heat production. - How does the electron transport chain connect to other metabolic pathways?
It integrates with glycolysis, the citric acid cycle, beta-oxidation, and amino acid metabolism, using their products to drive ATP synthesis.
By providing detailed answers to these frequently asked questions, readers can gain a deeper understanding of the electron transport chain and its importance in cellular energy metabolism.
Are you looking for comprehensive and reliable information on the latest trends, analyses, and solutions in the transportation industry? Visit worldtransport.net today to explore our in-depth articles and stay ahead in the ever-evolving world of transportation. Discover how we connect fundamental biological processes like the electron transport chain to the complexities of modern logistics, driving innovation and sustainability.

