Fermentation and respiration stand as the two primary mechanisms through which chemoheterotrophs produce ATP. While respiration harnesses the power of a proton gradient and oxidative phosphorylation for ATP synthesis, fermentation relies solely on substrate-level phosphorylation within metabolic pathways. Generally, fermentation yields less ATP compared to respiration. However, fermentation becomes essential or advantageous in certain scenarios, particularly when an appropriate final electron acceptor for respiration is absent. Many prokaryotes, including E. coli, are facultative anaerobes, capable of switching to respiration for greater ATP production if environmental conditions provide a suitable inorganic final electron acceptor. Conversely, some organisms, like members of Streptococcus and Clostridium, are obligate fermenters, entirely dependent on fermentation for ATP generation due to the lack of respiratory capabilities.
A crucial aspect of energy metabolism is the recycling of NADH back to NAD+. This regeneration is vital because NAD+ acts as an essential electron carrier in glycolysis and other catabolic pathways. When respiration is not an option, cells employ fermentation to reoxidize NADH. In this process, a metabolite produced by the cell, often pyruvate, serves as the final electron acceptor. Because the fundamental purpose of fermentation is to regenerate NAD+, the net NADH production in any fermentation pathway must be zero. Concurrently, to be energetically useful, fermentation pathways must also result in a net gain of ATP.
Fermentation: Bypassing the Electron Transport Chain
A defining characteristic of fermentation is its independence from an electron transport chain (ETC). Unlike respiration, fermentation does not involve an ETC and, consequently, does not directly generate any additional ATP beyond the substrate-level phosphorylation that occurs during glycolysis. Organisms that ferment typically achieve a maximum yield of only two ATP molecules per glucose molecule processed through glycolysis.
To understand this distinction better, let’s compare ATP synthesis methods and final electron acceptors across aerobic respiration, anaerobic respiration, and fermentation, as shown in the table below. It’s important to note that the ATP numbers for glycolysis assume the Embden-Meyerhof-Parnas (EMP) pathway. The table clearly distinguishes between ATP produced via substrate-level phosphorylation (SLP) and oxidative phosphorylation (OP).
| Type of Metabolism | Example | Final Electron Acceptor | Pathways Involved in ATP Synthesis (Type of Phosphorylation) | Maximum Yield of ATP Molecules |
|---|---|---|---|---|
| Aerobic respiration | Pseudomonas aeruginosa | O2 | EMP glycolysis (SLP) Krebs cycle (SLP) Electron transport and chemiosmosis (OP): | 2 2 34 |
| Total | 38 | |||
| Anaerobic respiration | Paracoccus denitrificans | NO3−,SO−24,Fe+3 other inorganics | EMP glycolysis (SLP) Krebs cycle (SLP) Electron transport and chemiosmosis (OP): | 2 2 1–32 |
| Total | 5–36 | |||
| Fermentation | Candida albicans | Organics (usually pyruvate) | EMP glycolysis (SLP) Fermentation | 2 0 |
| Total | 2 |
In essence, fermentation is an anaerobic process that regenerates NAD+ without the need for oxygen or an electron transport chain. This is a critical distinction from respiration, where the ETC plays a central role in ATP generation.
Common Fermentation Pathways and Their Significance
Bacterial fermentations frequently produce organic acids as waste products, a feature widely used in bacterial identification through metabolic tests. For instance, E. coli ferments lactose and produces gas, whereas some related Gram-negative bacteria do not. Similarly, sorbitol fermentation helps identify the pathogenic E. coli O157:H7 strain, as it cannot ferment sorbitol unlike other E. coli strains. Mannitol fermentation differentiates Staphylococcus aureus, a mannitol fermenter, from other non-fermenting staphylococci.
Lactic Acid Fermentation
The simplest fermentation type is homolactic or lactic acid fermentation (Figure 1), employed by bacteria in yogurt and soured foods, and by animal muscles during oxygen depletion. In homolactic fermentation, NADH electrons from glycolysis are transferred to pyruvate, the end product of glycolysis, to regenerate NAD+. This process yields lactate (lactic acid) as the sole waste product.
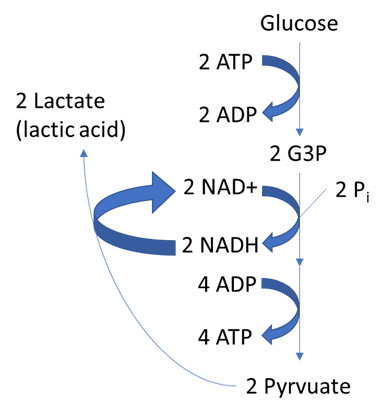 clipboard_e3044b526f495241b2bdf4534f1a655b4.png
clipboard_e3044b526f495241b2bdf4534f1a655b4.png
Bacteria like Lactobacillus, Leuconostoc, and Streptococcus, known as lactic acid bacteria (LAB), are vital in food production. Lactic acid fermentation during yogurt and cheese making acidifies the environment, denaturing milk proteins and causing solidification. When lactic acid is the only product, it’s homolactic fermentation, as seen in Lactobacillus delbrueckii and S. thermophiles used in yogurt. However, heterolactic fermentation, used by many bacteria, produces a mix of lactic acid, ethanol, acetic acid, and CO2, often due to the use of the pentose phosphate pathway instead of the EMP pathway for glycolysis. Leuconostoc mesenteroides is a key heterolactic fermenter used in souring vegetables like cucumbers and cabbage for pickles and sauerkraut.
Lactic acid bacteria are also medically significant. They create low pH environments in the body, inhibiting pathogen colonization. For example, vaginal microbiota, rich in lactic acid bacteria, prevents yeast infections. Furthermore, lactic acid bacteria are crucial for gut health and are primary components of probiotics.
Alcohol Fermentation
Another well-known process is alcohol fermentation by yeast, resulting in ethanol (Figure 2). Unlike bacterial fermentations, this eukaryotic fermentation does not produce acid waste. Saccharomyces cerevisiae yeast ferments pyruvate into ethanol, essential for alcoholic beverage production and bread rising due to CO2 release. Beyond food, ethanol fermentation of plant matter is important in biofuel production.
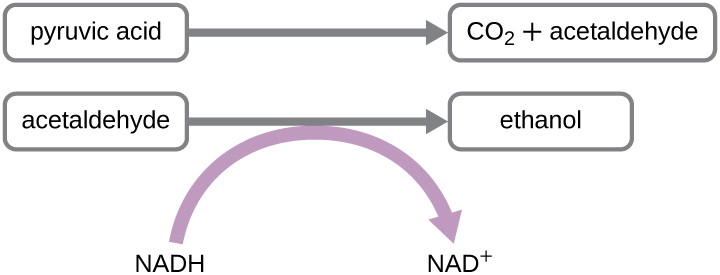 Pyruvic acid is converted to CO2 andacetaldehyde. Acetaldehyde is converted to ethanol; in this process NADH is converted to NAD+
Pyruvic acid is converted to CO2 andacetaldehyde. Acetaldehyde is converted to ethanol; in this process NADH is converted to NAD+
Besides lactic acid and alcohol fermentation, microbes use various other fermentation pathways to ensure NAD+ availability for glycolysis (Table 2). These pathways are essential because without NAD+ regeneration, glycolysis halts, and glucose breakdown for ATP ceases. Most fermentation types, except homolactic fermentation, produce gas, typically CO2 and/or hydrogen gas. These diverse fermentation pathways are also crucial in food production, each contributing unique organic acids that define the flavors of fermented foods. For example, propionic acid fermentation gives Swiss cheese its distinctive taste.
Fermentation products extend beyond the food industry. Chemical solvents like acetone and butanol are made through acetone-butanol-ethanol fermentation. Complex pharmaceuticals in antibiotics (e.g., penicillin), vaccines, and vitamins are produced via mixed acid fermentation.
Fermentation in Bacterial Identification
Fermentation abilities and products are valuable for differentiating bacteria in laboratories for diagnostic purposes. Enteric bacteria, known for mixed acid fermentation, lower pH, detectable with pH indicators. Acetoin production during butanediol fermentation is also detectable. Gas production from fermentation can be observed in Durham tubes, which trap gas in broth cultures.
| Pathway | End Products | Example Microbes | Commercial Products |
|---|---|---|---|
| Acetone-butanol-ethanol | Acetone, butanol, ethanol, CO2 | Clostridium acetobutylicum | Commercial solvents, gasoline alternative |
| Alcohol | Ethanol, CO2 | Candida, Saccharomyces | Beer, bread |
| Butanediol | Formic and lactic acid; ethanol; acetoin; 2,3 butanediol; CO2; hydrogen gas | Klebsiella, Enterobacter | Chardonnay wine |
| Butyric acid | Butyric acid, CO2, hydrogen gas | Clostridium butyricum | Butter |
| Lactic acid | Lactic acid | Streptococcus, Lactobacillus | Sauerkraut, yogurt, cheese |
| Mixed acid | Acetic, formic, lactic, and succinic acids; ethanol, CO2, hydrogen gas | Escherichia, Shigella | Vinegar, cosmetics, pharmaceuticals |
| Propionic acid | Acetic acid, propionic acid, CO2 | Propionibacterium, Bifidobacterium | Swiss cheese |
Table 2: Common Fermentation Pathways
Conclusion: Fermentation and the Absence of the Electron Transport Chain
In summary, fermentation is a metabolic strategy that allows cells to produce ATP in the absence of a final inorganic electron acceptor and without using an electron transport chain. It is characterized by substrate-level phosphorylation and the use of an organic molecule, often pyruvate or a pyruvate derivative, as the final electron acceptor. This process regenerates NAD+ necessary for glycolysis to continue, albeit with a lower ATP yield compared to respiration. Despite its lower energy efficiency, fermentation is vital for many microorganisms and has significant industrial and diagnostic applications. From producing flavorful foods and biofuels to enabling bacterial identification, fermentation plays a crucial role in both natural and industrial processes.
