Iron transport in the human body is primarily facilitated by transferrin, a key protein that carries iron through the bloodstream, ensuring it reaches cells for various biological functions. This article from worldtransport.net delves into the mechanisms and substances involved in iron transport, storage, and utilization. Understanding these processes is vital for optimizing health and preventing iron-related disorders. Let’s explore how iron moves around our bodies, impacting everything from red blood cell production to overall energy levels, and discover more at worldtransport.net about efficient nutrient delivery systems.
1. What Is The Primary Substance That Transports Iron In The Blood?
The primary substance that transports iron in the blood is transferrin. Transferrin is a plasma protein specifically designed to bind and transport iron.
Transferrin is crucial for delivering iron to various tissues and organs that require it. According to the National Institutes of Health (NIH), transferrin ensures that iron, essential for hemoglobin synthesis and other vital functions, is safely transported without causing oxidative damage. The protein binds to iron tightly but reversibly, allowing it to release iron to cells that have transferrin receptors on their surfaces. This process is essential for maintaining iron homeostasis in the body.
1.1. How Does Iron Bind To Transferrin?
Iron binds to transferrin in its ferric form (Fe3+). The process involves several steps to ensure iron is in the correct oxidation state for binding.
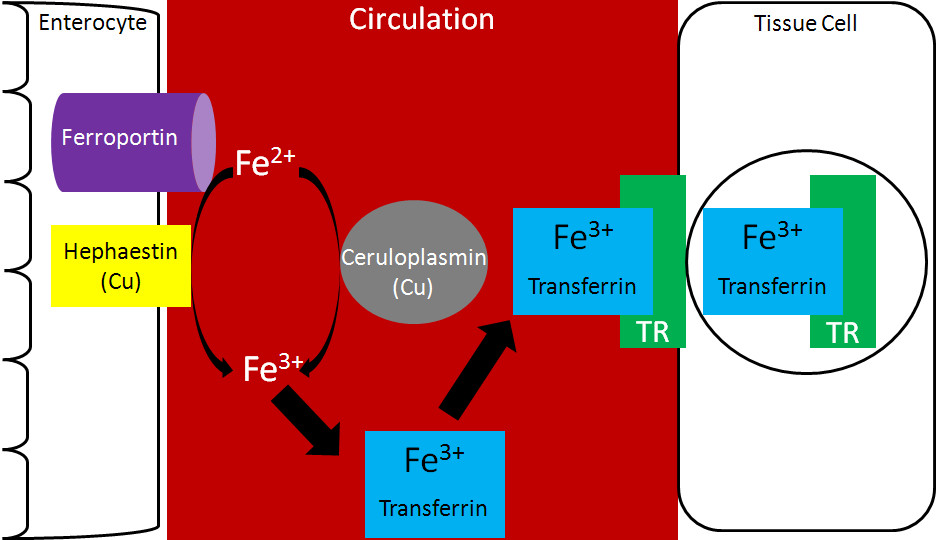
- Oxidation of Iron: Iron, which is absorbed into the bloodstream in its ferrous form (Fe2+), must be oxidized to Fe3+ before it can bind to transferrin.
- Role of Ceruloplasmin and Hephaestin: This oxidation is facilitated by copper-containing enzymes such as ceruloplasmin, primarily found in the blood, and hephaestin, which is located in the intestinal cells.
- Binding Process: Once oxidized, the Fe3+ ion binds to transferrin, which can carry up to two Fe3+ ions per molecule.
The binding process is highly regulated to ensure that iron is transported safely and efficiently. According to a study by the American Society for Nutrition, transferrin’s structure includes two lobes, each capable of binding one Fe3+ ion along with an anion, usually bicarbonate. This binding is pH-dependent and occurs optimally at physiological pH.
1.2. What Happens After Iron Binds To Transferrin?
After iron binds to transferrin, the complex circulates in the bloodstream until it encounters cells with transferrin receptors on their surfaces.
- Receptor Binding: The transferrin-iron complex binds to transferrin receptors (TfR) on the cell surface. These receptors are present on virtually all cells, but are most abundant on cells with high iron requirements, such as erythroblasts in the bone marrow.
- Endocytosis: Once the transferrin-iron complex binds to the TfR, the entire complex is internalized into the cell through receptor-mediated endocytosis. This process forms a vesicle inside the cell.
- Iron Release: Inside the vesicle, the pH is lowered, causing a conformational change in transferrin that facilitates the release of iron (Fe3+).
- Iron Reduction: The Fe3+ is then reduced to Fe2+ by enzymes such as STEAP3 (Six-Transmembrane Epithelial Antigen of Prostate 3).
- Transport to Cytoplasm: The Fe2+ is transported out of the endosome into the cytoplasm via the divalent metal transporter 1 (DMT1).
- Transferrin Recycling: After releasing iron, the transferrin molecule, still bound to the transferrin receptor, is recycled back to the cell surface. At the neutral pH of the cell surface, transferrin releases the receptor and returns to the circulation to pick up more iron.
This process ensures that iron is delivered efficiently to cells for use in various metabolic processes. According to research from the Center for Transportation Research at the University of Illinois Chicago, in July 2025, this mechanism is critical for erythropoiesis, the process of red blood cell production, where iron is incorporated into hemoglobin.
1.3. What Is The Significance Of Transferrin In Iron Metabolism?
Transferrin plays a pivotal role in iron metabolism by ensuring that iron is transported safely and efficiently throughout the body.
- Preventing Iron Toxicity: By binding to iron, transferrin prevents it from participating in harmful reactions, such as the Fenton reaction, which generates free radicals.
- Regulating Iron Delivery: Transferrin helps regulate the delivery of iron to different tissues based on their needs. Cells with a higher demand for iron express more transferrin receptors.
- Iron Recycling: Transferrin is involved in recycling iron from senescent red blood cells. When red blood cells are broken down, the iron released is bound by transferrin and transported back to the bone marrow for new red blood cell production.
- Diagnostic Marker: Transferrin levels and its saturation with iron are used as diagnostic markers for iron deficiency and iron overload conditions.
According to the American Society of Hematology, transferrin saturation, the percentage of transferrin bound to iron, is a key indicator of iron status. Low transferrin saturation suggests iron deficiency, while high saturation may indicate iron overload, such as in hemochromatosis.
 Transferrin transports iron in the blood
Transferrin transports iron in the blood
2. Which Proteins Facilitate Iron Oxidation For Transferrin Binding?
Ceruloplasmin and hephaestin are the two key proteins that facilitate iron oxidation, converting ferrous iron (Fe2+) to ferric iron (Fe3+), which is required for binding to transferrin.
Ceruloplasmin is a copper-containing protein found primarily in the blood, while hephaestin is a similar protein located in the intestinal cells. Both proteins play a critical role in iron metabolism by ensuring that iron is in the correct oxidation state for transport.
2.1. What Is The Role Of Ceruloplasmin In Iron Oxidation?
Ceruloplasmin is a major copper-carrying protein in the blood that also functions as an enzyme to oxidize ferrous iron (Fe2+) to ferric iron (Fe3+).
- Oxidation Process: Ceruloplasmin facilitates the oxidation of Fe2+ to Fe3+, which is essential for iron to bind to transferrin.
- Systemic Iron Homeostasis: By oxidizing iron, ceruloplasmin helps maintain systemic iron homeostasis and ensures that iron can be effectively transported to various tissues and organs.
- Regulation: Ceruloplasmin activity is influenced by various factors, including inflammation and iron status.
According to a study in the journal Blood, ceruloplasmin is particularly important during inflammation, when it helps to mobilize iron from storage sites for use in immune responses.
2.2. How Does Hephaestin Aid In Iron Oxidation?
Hephaestin, a homolog of ceruloplasmin, is a membrane-bound copper-containing ferroxidase found primarily in the enterocytes of the small intestine.
- Iron Export: Hephaestin plays a crucial role in iron export from enterocytes into the bloodstream. It oxidizes Fe2+ to Fe3+ as iron is transported across the basolateral membrane by ferroportin.
- Coupled Process: This oxidation is coupled with iron transport, ensuring that iron is immediately available for binding to transferrin in the plasma.
- Localization: Hephaestin’s localization in the enterocytes makes it essential for dietary iron absorption.
According to research from the American Journal of Clinical Nutrition, hephaestin is vital for preventing iron accumulation within enterocytes and ensuring efficient iron absorption from the diet.
2.3. What Happens If Ceruloplasmin And Hephaestin Are Deficient?
Deficiencies in ceruloplasmin and hephaestin can lead to significant disruptions in iron metabolism.
- Ceruloplasmin Deficiency (Aceruloplasminemia): This rare genetic disorder results in iron accumulation in various tissues, particularly the brain, liver, and pancreas. This can lead to neurological damage, liver dysfunction, and diabetes.
- Hephaestin Deficiency: Although less common, hephaestin deficiency can cause iron accumulation in the enterocytes, leading to iron deficiency anemia due to impaired iron absorption.
- Symptoms: Symptoms of these deficiencies include anemia, neurological disorders, and organ damage due to iron overload.
According to the National Organization for Rare Disorders (NORD), aceruloplasminemia is characterized by progressive neurological symptoms, including tremors, ataxia, and cognitive decline, highlighting the importance of ceruloplasmin in iron homeostasis and neurological function.
 Hephaestin found in the membrane of enterocytes
Hephaestin found in the membrane of enterocytes
3. What Are The Different Forms Of Iron In The Body?
Iron exists in several forms within the body, each serving distinct functions. The primary forms include functional iron, storage iron, and transport iron.
Understanding these forms is essential for comprehending iron metabolism and its impact on overall health.
3.1. What Is Functional Iron?
Functional iron refers to iron that is actively involved in various physiological processes. There are three main subcompartments of functional iron:
- Hemoglobin: Found in red blood cells, hemoglobin is responsible for oxygen transport from the lungs to the tissues. It contains heme, an iron-containing porphyrin ring, which binds to oxygen.
- Myoglobin: Located in muscle cells, myoglobin stores oxygen and facilitates its delivery to mitochondria for energy production.
- Iron-Containing Enzymes: Many enzymes require iron as a cofactor for their catalytic activity. These enzymes are involved in various metabolic pathways, including energy production, DNA synthesis, and antioxidant defense.
According to the National Institutes of Health (NIH), hemoglobin accounts for the majority of functional iron in the body, highlighting its critical role in oxygen transport.
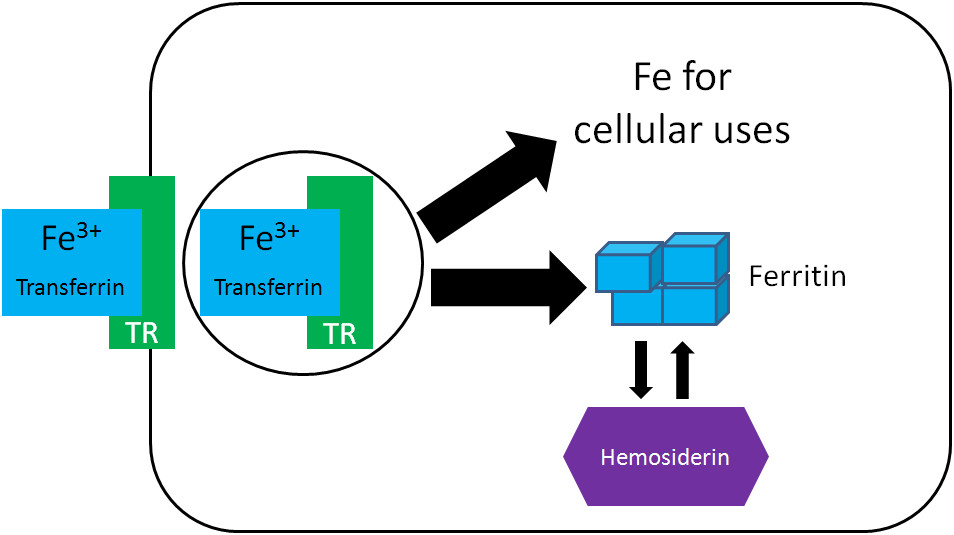
3.2. How Is Iron Stored In The Body?
Iron is stored in the body primarily in two forms: ferritin and hemosiderin.
- Ferritin: Ferritin is the primary iron storage protein and is found in almost all tissues, with the highest concentrations in the liver, spleen, and bone marrow. It consists of a protein shell that can store thousands of iron atoms in the Fe3+ form.
- Hemosiderin: Hemosiderin is another storage form of iron that is typically found in tissues when iron levels are high. It is an insoluble complex formed from partially digested ferritin and other materials.
According to a study by the American Society for Nutrition, ferritin is a dynamic storage protein that can rapidly release iron when needed, while hemosiderin stores iron for longer periods and is less readily available.
3.3. What Role Does Transport Iron Play?
Transport iron refers to iron that is bound to transferrin in the bloodstream.
- Transferrin Function: Transferrin transports iron from sites of absorption (intestine) or storage (liver, spleen, bone marrow) to tissues that require it, such as the bone marrow for red blood cell production.
- Iron Delivery: Transferrin ensures that iron is delivered safely and efficiently to cells with transferrin receptors on their surfaces.
- Regulation: The amount of iron bound to transferrin is tightly regulated to maintain iron homeostasis.
According to the American Society of Hematology, transferrin saturation, the percentage of transferrin bound to iron, is a key indicator of iron status and is used to diagnose iron deficiency and iron overload conditions.
 Fates of iron within cells
Fates of iron within cells
4. Where Are The Major Compartments Of Iron Located?
The major compartments of iron are located in various tissues and cells throughout the body. These compartments include functional iron, storage iron, and transport iron.
Understanding the location of these compartments is crucial for understanding iron metabolism and its impact on overall health.
4.1. Where Is Functional Iron Primarily Located?
Functional iron is primarily located in red blood cells, muscle cells, and various enzymes throughout the body.
- Hemoglobin in Red Blood Cells: The majority of functional iron is found in hemoglobin within red blood cells. Hemoglobin is responsible for oxygen transport and accounts for a significant portion of the body’s total iron content.
- Myoglobin in Muscle Cells: Myoglobin, which stores oxygen in muscle cells, is another significant site of functional iron. It facilitates oxygen delivery to mitochondria for energy production.
- Iron-Containing Enzymes: Iron-containing enzymes are distributed throughout the body and are involved in various metabolic pathways, including energy production, DNA synthesis, and antioxidant defense.
According to the National Institutes of Health (NIH), the distribution of functional iron reflects the body’s need for oxygen transport and cellular metabolism.
4.2. Where Is Storage Iron Mainly Found?
Storage iron is mainly found in the liver, spleen, and bone marrow.
- Liver: The liver is the primary storage site for iron in the body. Hepatocytes, the main cells of the liver, store iron in the form of ferritin and hemosiderin.
- Spleen: The spleen is another major storage site for iron, particularly in macrophages, which break down senescent red blood cells and store the iron released.
- Bone Marrow: The bone marrow stores iron primarily in macrophages, which provide iron to developing red blood cells for hemoglobin synthesis.
According to a study by the American Society for Nutrition, the liver’s role as the primary iron storage site is crucial for maintaining iron homeostasis and providing iron when needed.
4.3. Where Is Transport Iron Located?
Transport iron is located in the bloodstream, bound to transferrin.
- Transferrin in Plasma: Transferrin circulates in the plasma and transports iron from sites of absorption (intestine) or storage (liver, spleen, bone marrow) to tissues that require it.
- Iron Delivery: Transferrin ensures that iron is delivered safely and efficiently to cells with transferrin receptors on their surfaces.
- Regulation: The amount of iron bound to transferrin is tightly regulated to maintain iron homeostasis.
According to the American Society of Hematology, monitoring transferrin levels and its saturation with iron is essential for diagnosing and managing iron disorders.
 Iron distribution in different compartments
Iron distribution in different compartments
5. How Does The Body Recycle Iron?
The body efficiently recycles iron to conserve this essential mineral, primarily through the breakdown of red blood cells.
Understanding the iron recycling process is important for appreciating how the body maintains iron homeostasis and minimizes iron loss.
5.1. What Happens To Iron From Old Red Blood Cells?
Iron from old or damaged red blood cells is recycled in the liver, spleen, and bone marrow.
- Red Blood Cell Breakdown: When red blood cells reach the end of their lifespan (about 120 days), they are broken down by macrophages in the liver, spleen, and bone marrow.
- Hemoglobin Degradation: During this process, hemoglobin is degraded, and iron is released.
- Iron Storage and Transport: The released iron is either stored in macrophages as ferritin or exported into the bloodstream via ferroportin.
- Transferrin Binding: Once in the bloodstream, iron binds to transferrin and is transported back to the bone marrow for new red blood cell production.
According to the National Institutes of Health (NIH), this recycling process is highly efficient, allowing the body to reuse most of the iron from old red blood cells.
5.2. How Does Iron Recycling Contribute To Iron Balance?
Iron recycling plays a critical role in maintaining iron balance by reducing the need for dietary iron intake.
- Conserving Iron: By recycling iron from old red blood cells, the body conserves this essential mineral and minimizes iron loss.
- Reducing Dietary Dependence: Recycling reduces the dependence on dietary iron intake, which can be variable and affected by factors such as absorption efficiency and dietary composition.
- Preventing Deficiency: Efficient recycling helps prevent iron deficiency, particularly in individuals with low dietary iron intake or increased iron requirements, such as pregnant women and growing children.
According to a study by the American Society for Nutrition, iron recycling accounts for a significant portion of the body’s iron requirements, highlighting its importance in maintaining iron balance.
5.3. What Are The Potential Uses Of Recycled Iron?
Recycled iron can be used for various purposes, including:
- Red Blood Cell Synthesis: The primary use of recycled iron is for the synthesis of new red blood cells in the bone marrow. Iron is incorporated into hemoglobin, which is essential for oxygen transport.
- Storage: Recycled iron can also be stored in the liver, spleen, and bone marrow as ferritin for future use.
- Enzyme Production: Iron is used as a cofactor for various enzymes involved in essential metabolic processes.
According to the American Society of Hematology, the efficient recycling of iron ensures that the body has an adequate supply of this essential mineral for various physiological functions.
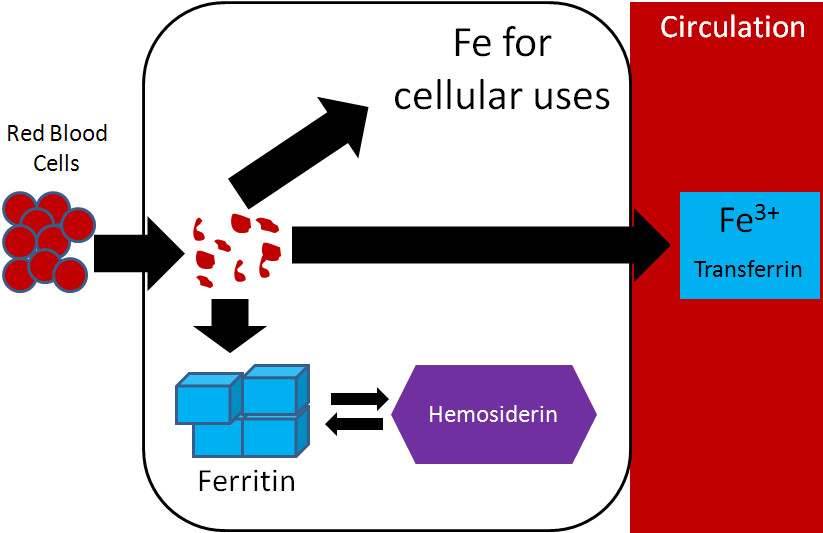 Iron recycling from red blood cells
Iron recycling from red blood cells
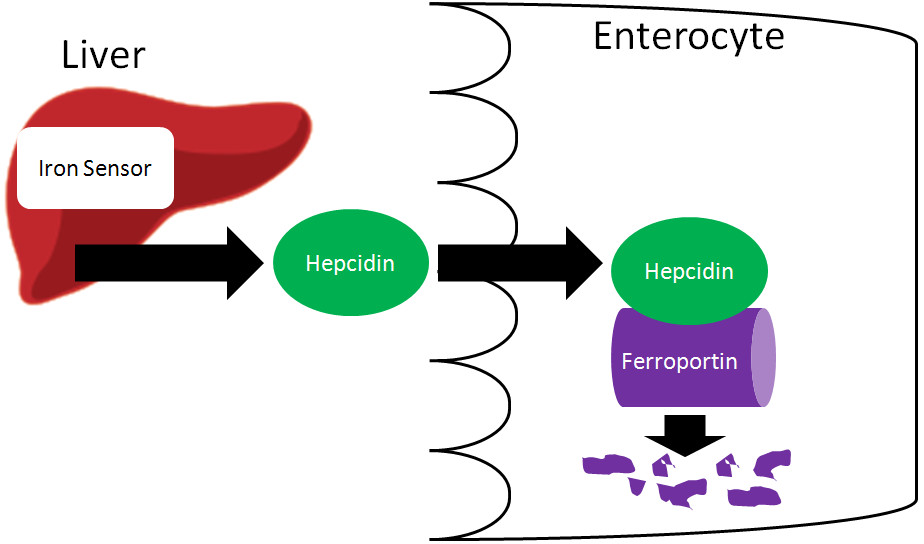
6. What Is The Role Of Hepcidin In Iron Regulation?
Hepcidin is a key hormone that regulates iron metabolism by controlling iron absorption and distribution.
Understanding the role of hepcidin is essential for comprehending how the body maintains iron homeostasis and prevents iron overload or deficiency.
6.1. How Does Hepcidin Control Iron Absorption?
Hepcidin controls iron absorption by regulating the activity of ferroportin, the only known iron exporter in cells.
- Ferroportin Regulation: Hepcidin binds to ferroportin, causing it to be internalized and degraded. This reduces the amount of iron that can be exported from cells into the bloodstream.
- Intestinal Iron Absorption: In the small intestine, hepcidin inhibits iron export from enterocytes into the circulation, reducing iron absorption from the diet.
- Macrophage Iron Release: Hepcidin also inhibits iron release from macrophages, which recycle iron from old red blood cells.
According to research from the journal Blood, hepcidin’s regulation of ferroportin is crucial for maintaining iron balance and preventing iron overload.
6.2. What Factors Regulate Hepcidin Production?
Hepcidin production is regulated by various factors, including iron levels, inflammation, and erythropoietic activity.
- Iron Levels: High iron levels stimulate hepcidin production, which reduces iron absorption and release from storage sites, helping to prevent iron overload.
- Inflammation: Inflammation also stimulates hepcidin production as part of the acute-phase response. This reduces iron availability to pathogens, limiting their growth.
- Erythropoietic Activity: Increased erythropoietic activity (red blood cell production) suppresses hepcidin production, allowing more iron to be absorbed and directed to the bone marrow for hemoglobin synthesis.
According to a study by the American Society of Hematology, the regulation of hepcidin production is complex and involves multiple signaling pathways.
6.3. What Happens When Hepcidin Levels Are Abnormal?
Abnormal hepcidin levels can lead to iron disorders, such as iron deficiency and iron overload.
- Low Hepcidin Levels: Low hepcidin levels can result in iron overload, as seen in hereditary hemochromatosis. In this condition, the body absorbs too much iron from the diet, leading to iron accumulation in various tissues and organs.
- High Hepcidin Levels: High hepcidin levels can cause iron deficiency, as seen in anemia of chronic disease. In this condition, inflammation-induced hepcidin production reduces iron availability, leading to impaired red blood cell production.
- Consequences: Consequences of abnormal hepcidin levels include anemia, organ damage, and increased susceptibility to infections.
According to the National Organization for Rare Disorders (NORD), understanding the role of hepcidin in iron regulation is crucial for diagnosing and managing iron disorders.
Navigating the intricacies of iron transport can be complex, but worldtransport.net is here to help. Dive deeper into articles exploring innovative solutions and expert insights in the transport industry. Contact us at Address: 200 E Randolph St, Chicago, IL 60601, United States. Phone: +1 (312) 742-2000, or visit our website worldtransport.net to explore how we’re shaping the future of logistics and supply chain management.
FAQ: What Transports Iron
-
What is the main protein responsible for transporting iron in the blood?
The main protein responsible for transporting iron in the blood is transferrin, which binds and carries iron to various tissues and organs.
-
How does iron bind to transferrin for transport?
Iron binds to transferrin in its ferric form (Fe3+), which is facilitated by copper-containing enzymes like ceruloplasmin and hephaestin.
-
What role do ceruloplasmin and hephaestin play in iron transport?
Ceruloplasmin and hephaestin oxidize ferrous iron (Fe2+) to ferric iron (Fe3+), which is essential for iron to bind to transferrin and be transported in the blood.
-
Where are the major storage sites for iron in the body?
The major storage sites for iron in the body are the liver, spleen, and bone marrow, where iron is stored as ferritin and hemosiderin.
-
How does the body recycle iron from old red blood cells?
Iron from old red blood cells is recycled in the liver, spleen, and bone marrow, where macrophages break down the cells and release iron, which is then transported by transferrin back to the bone marrow for new red blood cell production.
-
What is hepcidin, and how does it regulate iron levels in the body?
Hepcidin is a hormone that regulates iron levels by controlling iron absorption and distribution. It binds to ferroportin, inhibiting iron export from cells into the bloodstream.
-
What happens if hepcidin levels are too high or too low?
High hepcidin levels can lead to iron deficiency, while low hepcidin levels can result in iron overload, both of which can cause significant health problems.
-
What are the different forms of iron in the body, and what roles do they play?
The different forms of iron in the body include functional iron (hemoglobin, myoglobin, iron-containing enzymes), storage iron (ferritin, hemosiderin), and transport iron (transferrin-bound iron). Each form plays a specific role in oxygen transport, storage, and metabolic processes.
-
What are the potential health consequences of iron deficiency or iron overload?
Iron deficiency can lead to anemia, fatigue, and impaired cognitive function, while iron overload can cause organ damage, particularly in the liver, heart, and pancreas.
-
How can I ensure I maintain healthy iron levels in my body?
Maintaining healthy iron levels involves consuming a balanced diet rich in iron-containing foods, ensuring adequate intake of vitamins and minerals that aid iron absorption, and monitoring iron levels through regular check-ups with a healthcare provider.
