The electron transport chain (ETC) is a fundamental process for life as we know it, acting as the linchpin of cellular respiration. This intricate series of biochemical reactions is responsible for generating the majority of ATP, the energy currency of the cell, through a process called oxidative phosphorylation. But Where Does Electron Transport Occur within the cell? The answer, while seemingly simple, reveals a fascinating story of cellular evolution and adaptation, differing significantly between eukaryotes and prokaryotes. Understanding the location of the ETC is crucial to grasping how cells efficiently convert energy from nutrients into a usable form.
Electron Transport Chain in Eukaryotes: Mitochondria – The Powerhouse
In eukaryotic organisms, including animals, plants, fungi, and protists, the electron transport chain is located within specialized organelles called mitochondria. Often referred to as the “powerhouses of the cell,” mitochondria are double-membrane-bound structures that are the primary sites of ATP production.
Inner Mitochondrial Membrane: The Stage for ETC
Specifically, the eukaryotic electron transport chain resides in the inner mitochondrial membrane. This membrane is highly folded into cristae, which significantly increase the surface area available for the ETC machinery. Embedded within this membrane are a series of protein complexes, namely Complexes I, II, III, and IV, along with mobile electron carriers like coenzyme Q and cytochrome c. These components work in concert to facilitate the step-wise transfer of electrons.
As illustrated in Figure 1, electrons derived from NADH and FADH2, generated during earlier stages of cellular respiration like glycolysis and the Krebs cycle, enter the ETC. These electrons are passed from one complex to the next in a series of redox reactions. This electron flow is coupled with the pumping of protons (H+) from the mitochondrial matrix to the intermembrane space, creating an electrochemical gradient.
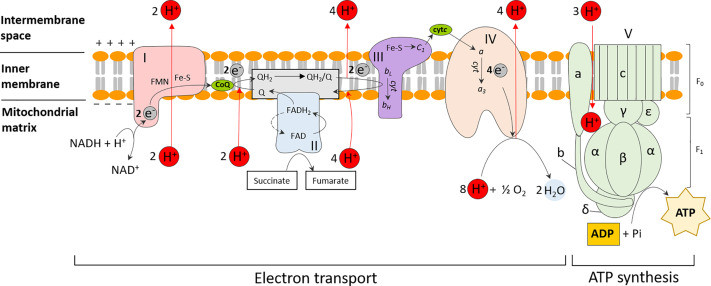 Diagram illustrating oxidative phosphorylation in eukaryotes, showing the electron transport chain complexes (I-V) within the inner mitochondrial membrane, ATP synthase, and the movement of protons to create a proton motive force for ATP synthesis. Key components like NADH, FADH2, CoQ, and cytochrome c are labeled.
Diagram illustrating oxidative phosphorylation in eukaryotes, showing the electron transport chain complexes (I-V) within the inner mitochondrial membrane, ATP synthase, and the movement of protons to create a proton motive force for ATP synthesis. Key components like NADH, FADH2, CoQ, and cytochrome c are labeled.
Figure 1. Oxidative phosphorylation in eukaryotes. ATP synthesis is coupled to the sequential transfer of electrons via an electron transport chain from NADH or FADH2 to oxygen. This process is facilitated by a series of electron carriers, which also serve to establish the proton motive force by translocating protons outside the membrane. This generates an electrochemical gradient which fuels the final ATP synthesis step. Abbreviations are as follows: complex I, NADH coenzyme Q reductase; complex II, succinate dehydrogenase; complex III, cytochrome bc1; complex IV, cytochrome c oxidase; complex V, F0F1 ATP synthase; CoQ, coenzyme Q; cytC, cytochrome c. The subunits (a, b, c, α, β, δ, ε and γ) which make up the F0 and F1 units of complex V are also indicated.
Complex V, also known as ATP synthase, is the final major protein complex in this process. It utilizes the proton gradient established by the ETC to drive the synthesis of ATP. As protons flow back down their electrochemical gradient through ATP synthase, the energy released is harnessed to phosphorylate ADP, creating ATP. This process, known as chemiosmosis, is the culmination of the electron transport chain and the primary mechanism for ATP generation in aerobic eukaryotes.
Electron Transport Chain in Prokaryotes: Plasma Membrane – A Versatile Location
In contrast to eukaryotes, prokaryotic organisms, including bacteria and archaea, lack membrane-bound organelles like mitochondria. Therefore, in prokaryotes, the electron transport chain is located in the plasma membrane (also known as the cell membrane). This membrane serves as the barrier between the cell’s cytoplasm and the external environment.
Plasma Membrane: The Site of Prokaryotic ETC
The plasma membrane of prokaryotes houses all the necessary components for electron transport and oxidative phosphorylation, analogous to the inner mitochondrial membrane in eukaryotes. Similar to eukaryotes, prokaryotic ETCs involve a series of membrane-bound protein complexes and mobile electron carriers that facilitate redox reactions and proton pumping.
However, prokaryotic ETCs exhibit greater diversity compared to their eukaryotic counterparts. Bacteria and archaea can utilize a wider range of electron donors and acceptors, reflecting their adaptation to diverse environments. For instance, while eukaryotes primarily use oxygen as the final electron acceptor in aerobic respiration, prokaryotes can employ alternative acceptors such as sulfur, sulfate, nitrate, or nitrite in anaerobic respiration. This metabolic flexibility is reflected in the varied composition of their electron transport chains.
Variations in Prokaryotic ETC Location
While the plasma membrane is the primary location, the exact arrangement and components can vary significantly across different prokaryotic species. Some bacteria may have specialized regions within their plasma membrane that are enriched in ETC components. Furthermore, the specific dehydrogenases and oxidases involved can differ depending on the organism’s energy source and environmental conditions. For example, bacteria utilizing inorganic electron donors like nitrite or ferrous iron may have ETCs adapted to accept electrons at different points in the chain.
The Significance of Location in ETC Function
The membrane-bound location of the electron transport chain, whether in the inner mitochondrial membrane of eukaryotes or the plasma membrane of prokaryotes, is critical for its function. The compartmentalization provided by the membrane is essential for establishing and maintaining the proton gradient. This gradient, also known as the proton motive force, is the direct source of energy for ATP synthesis via chemiosmosis.
By confining the ETC to a membrane, cells can effectively separate the regions of high and low proton concentration, creating a reservoir of potential energy. This controlled environment allows for the efficient conversion of redox energy into the chemical energy of ATP. The strategic location ensures that the energy released during electron transport is not dissipated but rather harnessed to drive the crucial process of oxidative phosphorylation.
Conclusion: Location is Key to Cellular Respiration
In summary, where does electron transport occur? In eukaryotes, the electron transport chain is located in the inner mitochondrial membrane, within the specialized organelles called mitochondria. In prokaryotes, it resides in the plasma membrane. Despite the difference in location, the fundamental principle remains the same: the ETC is membrane-bound to facilitate the generation of a proton gradient, which is then used by ATP synthase to produce ATP. This strategic localization is a testament to the elegant and efficient design of cellular energy production, highlighting the fundamental importance of membrane systems in biological energy conversion across all forms of life.
