Introduction
Survival in the animal kingdom hinges on the ability to maintain a stable internal environment, a state known as homeostasis, despite constant fluctuations in both the external and internal surroundings. This delicate balance is orchestrated by intricate communication networks within and between cells across various tissues. Among the organ systems crucial for this communication and homeostatic maintenance, the nervous system stands out as a key player. This article delves into the multifaceted roles of neuronal inputs and outputs in pathways that influence aging and longevity, emphasizing how sensory information modulates lifespan through diverse neuronal signals. We will explore feedback, compensatory, and feed-forward mechanisms within these longevity pathways, all essential for upholding homeostasis. Furthermore, we consider the temporal dynamics of these neuronal processes and the potential influence of natural genetic variations in shaping the neurobiology of aging.
The study of aging is inherently the study of an open system. Throughout an animal’s life, there is a constant exchange of information between its tissues and organs, as well as with the external environment. These exchanges are crucial for maintaining homeostasis, a stable internal milieu necessary for survival in the face of environmental variability. The nervous system acts as a critical interface in this information flow, bridging the external and internal worlds of an organism. It is therefore not surprising that neuronal signaling and its regulation have a profound impact on an animal’s survival and the aging process itself. Here, we explore the nervous system’s role in maintaining homeostasis and its subsequent effects on longevity and the aging trajectory.
Signaling Networks: Intracellular, Intercellular, and Interorgan Communication in Homeostatic Maintenance and Lifespan
The nervous system is an intricate network of specialized cells designed to relay information between different organ systems and the external environment. Sensory neurons are responsible for perceiving environmental cues. This sensory information is then transmitted to non-neuronal tissues, either directly or indirectly, through neural circuits composed of interneurons and other neuron types, such as motor neurons. This intercellular and interorgan communication relies on a diverse array of signaling molecules, ranging from small molecule neurotransmitters to neuropeptides and hormones (Figure 1; reviewed in Alcedo et al., 2010). Crucially, and consistent with the understanding that the nervous system impacts longevity, the processing of environmental information by sensory neurons and the related neural circuits can modulate hormonal secretions that are vital for maintaining homeostasis (Figure 1; reviewed in Alcedo et al., 2010).
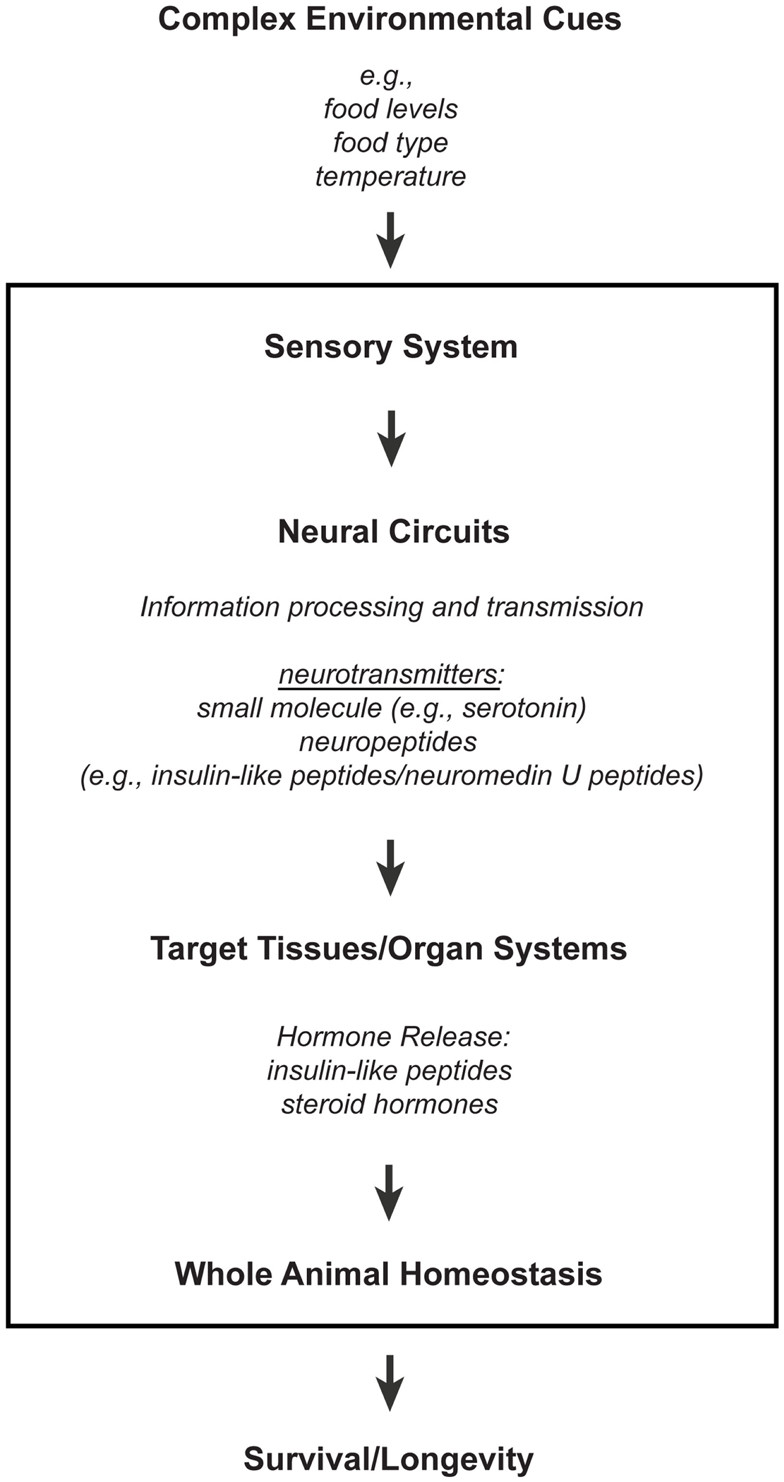 A model for how the nervous system processes environmental information through neuronal and non-neuronal circuits to maintain homeostasis for optimal survival
A model for how the nervous system processes environmental information through neuronal and non-neuronal circuits to maintain homeostasis for optimal survival
Figure 1. Neuronal processing of environmental cues for homeostasis. This model illustrates how the nervous system integrates environmental information through both neuronal and non-neuronal circuits to maintain homeostasis for optimal survival. Information processing of environmental inputs at the neuronal level involves the function of (1) small molecule neurotransmitters and neuropeptides, such as insulin-like peptides, (2) stress-sensing pathways, and (3) mitochondria-associated signals. These neuronal signaling outputs subsequently target other tissues to regulate the production of secondary signals, like hormones, thus promoting homeostasis and longevity. Insulin-like peptides can function as short-range peptide neurotransmitters (Chen et al., 2013) or as peptide hormones.
Sensory Influence on Homeostasis and Lifespan
Sensory perception has the remarkable ability to alter numerous physiological processes, from regulating circadian rhythms (Wurtman et al., 1963, 1964; la Fleur et al., 2001; Challet et al., 2003; Ha et al., 2006) and developmental plasticity (Bargmann and Horvitz, 1991; Schackwitz et al., 1996) to metabolism (Zafra et al., 2006; Greer et al., 2008), reproduction (Yoon et al., 2005), and stress responses (Prahlad et al., 2008). Furthermore, sensory neurons have been demonstrated to influence lifespan in the nematode worm C. elegans (Apfeld and Kenyon, 1999; Alcedo and Kenyon, 2004; Bishop and Guarente, 2007; Lee and Kenyon, 2009) and in the fruit fly Drosophila (Libert et al., 2007; Poon et al., 2010). This influence on lifespan can be either positive or negative, originating from gustatory, olfactory, and thermosensory neurons. These sensory inputs can modulate the activity of various peptide or steroid hormones (Apfeld and Kenyon, 1999; Alcedo and Kenyon, 2004; Libert et al., 2007; Lee and Kenyon, 2009), which in turn are presumed to affect diverse homeostatic mechanisms (reviewed in Fielenbach and Antebi, 2008; Kenyon, 2010). The detailed studies showcasing the sensory influence on C. elegans and Drosophila lifespan are comprehensively reviewed by Jeong et al. (2012).
The nature of some of these sensory neurons suggests that certain lifespan-influencing cues are derived from food. This aligns with the observation that olfactory inputs are involved in the lifespan effects of calorie restriction (Libert et al., 2007), a well-known phenomenon where reduced food intake promotes longevity (Klass, 1977; Weindruch and Walford, 1988). However, the longevity benefits of calorie restriction are often associated with changes in feeding rates, delayed development, and reduced reproduction (Klass, 1977; Weindruch and Walford, 1988). In contrast, the sensory influence on lifespan does not always correlate with the sensory effects on these behaviors (Apfeld and Kenyon, 1999; Alcedo and Kenyon, 2004; Poon et al., 2010). This suggests that the sensory system affects lifespan through multiple mechanisms, as different types of sensory neurons can perceive a wide array of environmental cues. These cues can range from temperature (Lee and Kenyon, 2009; Xiao et al., 2013) and the complexity of food sources (Libert et al., 2007; Maier et al., 2010; Poon et al., 2010) to numerous other factors, many of which have the potential to alter organismal homeostasis and impact lifespan.
Recent research has highlighted another form of dietary influence on lifespan mediated by the sensory system: the dependence on food-type or composition, distinct from the effects of food-level restriction (Maier et al., 2010). This is consistent with the earlier finding that only a specific subset of gustatory and olfactory neurons affects lifespan in a particular environment (Alcedo and Kenyon, 2004). In essence, the presence of specific lifespan-influencing cues in certain food sources will only be detected by a corresponding set of sensory neurons. This is further supported by the identification of a monocarboxylate-like transporter (MCT-1) that mediates the lifespan effects of only certain sensory neurons, suggesting that MCT-1 is selective in transporting small metabolites (Gaglia et al., 2012).
The sensory influence on lifespan through food-type recognition has also been linked to the activity of specific neuropeptide signaling pathways under particular environmental conditions (Maier et al., 2010). For example, the neuromedin U pathway processes food-type information that alters C. elegans lifespan, independently of food intake levels (Maier et al., 2010). Given that many species have a vast repertoire of neuropeptide ligands and receptors, many of which are expressed in the nervous system (Bargmann, 1998; Strand, 1999), these neuropeptide signaling pathways likely process distinct sets of sensory information into physiological responses that optimize survival.
Modulation of Lifespan and Aging by Neuronal Insulin/IGF Signaling
The sensory influence on lifespan can be mediated by insulin/insulin-like peptides (ILPs) and their associated signaling pathway, known as Insulin/IGF Signaling (IIS) (Apfeld and Kenyon, 1999; Alcedo and Kenyon, 2004). IIS is well-established as a central regulator of growth, development, metabolism, and reproduction. Among the molecular pathways known to affect longevity, IIS is arguably the most studied and significant, primarily due to its evolutionarily conserved and substantial impact on lifespan across various model organisms, from invertebrates to mammals (reviewed in Tatar et al., 2003; Taguchi and White, 2008; Partridge et al., 2011). Recent studies suggest that IIS action in the central nervous system (CNS) is particularly important for modulating aging and longevity (reviewed in Broughton and Partridge, 2009).
IIS in the CNS plays dual roles in aging. Firstly, it can exert local, neuroprotective effects within the CNS, such as promoting neuronal survival under neurodegenerative conditions (Chrysis et al., 2001; Schubert et al., 2004; Plum et al., 2005; Bateman and McNeill, 2006). Secondly, in response to environmental cues, including food-derived signals, CNS-acting factors can regulate the production and release of ILPs. These ILPs then act systemically to influence whole-organismal aging. Here we focus on these CNS-mediated, lifespan-promoting effects of reduced IIS in worms, flies, and mice (reviewed in Tatar et al., 2003; Fielenbach and Antebi, 2008; Alcedo et al., 2010).
The worm C. elegans possesses 40 genes predicted to encode ILPs, many of which are expressed in sensory neurons and interneurons and can function as ligands for the insulin receptor ortholog DAF-2 (Pierce et al., 2001; Li et al., 2003; Cornils et al., 2011). In line with the idea that sensory neurons produce and release ILPs that regulate lifespan through IIS in distant tissues, mutations causing defects in ciliated sensory neurons, or targeted ablation of gustatory and olfactory neurons, extend lifespan. This lifespan extension is fully or partially dependent on DAF-16/FOXO, a transcription factor downstream of IIS that is activated when IIS is reduced (Apfeld and Kenyon, 1999; Alcedo and Kenyon, 2004; Shen et al., 2010). The central role of the CNS in IIS modulation of longevity is further supported by the finding that the extended lifespan resulting from mutations in daf-2 and age-1/PI-3K, a key kinase downstream of DAF-2, can be largely or fully reversed when wild-type daf-2 or age-1 is expressed specifically in the neurons of the corresponding mutants (Wolkow et al., 2000; Iser et al., 2007). In contrast, neuronal activity of DAF-16/FOXO appears less critical for lifespan extension in animals with impaired IIS (Libina et al., 2003; Iser et al., 2007). However, the microRNA mir-71, when expressed in the nervous system, mediates lifespan extension in germline-ablated worms, and this process depends on intestinal DAF-16 activity. This reveals a complex signaling interaction between the CNS, intestine, and gonad in IIS-mediated lifespan regulation (Boulias and Horvitz, 2012).
Studies in the fruit fly Drosophila melanogaster reveal remarkable parallels to these findings in worms. In adult flies, three of the seven distinct ILPs are produced in specialized median neurosecretory cells (also known as insulin-producing cells, IPCs) in the pars intercerebralis of the CNS (Rulifson et al., 2002; Grönke et al., 2010). Ablation of these IPCs significantly extends lifespan (Wessells et al., 2004; Broughton et al., 2005; Haselton et al., 2010), likely due to reduced levels of ILP2, ILP3, and ILP5 (Broughton et al., 2008; Grönke et al., 2010). Consistent with these observations, several factors regulating ILP production and/or release affect IIS and lifespan. These include metabotropic GABA receptors or uncoupling proteins (UCPs) expressed in IPCs (Fridell et al., 2009; Humphrey et al., 2009; Enell et al., 2010), and short neuropeptide F (sNPF) expressed in the CNS (Lee et al., 2008, 2009). Furthermore, downregulation of p53 in IPCs extends lifespan by reducing ILP levels and inhibiting PI-3K activity in peripheral tissues (Bauer et al., 2007). Similarly, the stress-responsive Jun kinase (JNK) in IPCs promotes longevity by downregulating ILP2 through FOXO activation (Wang et al., 2005). Conversely, activation of FOXO in the CNS, whether pan-neuronally, in the neurolemma, or in glial cells, is not sufficient to extend lifespan. Instead, its downregulation in head fat body tissues promotes longevity (Hwangbo et al., 2004).
In mammals, the CNS also appears to play a crucial role in regulating the production and release of insulin-like hormones, although the majority of insulin or IGF-1 is produced outside the brain. For instance, mice with mutations affecting the hypothalamic-pituitary-somatotropic growth hormone (GH-IIS) axis, which regulates insulin/insulin-like hormone release, are long-lived, presumably due to IIS downregulation (reviewed in Tatar et al., 2003; Holzenberger et al., 2004; Berryman et al., 2008). More direct evidence for the nervous system’s role in IIS-mediated mammalian lifespan comes from studies with transgenic or mutant mice with impaired IIS. Mice with brain-specific deletion of the insulin receptor substrate-2 (Irs2) locus live 14% longer than controls, despite being hyperinsulinemic, obese, and insulin-resistant (Taguchi et al., 2007). Similarly, partial genetic inactivation of the IGF-1 receptor (IGF-1R) gene in the embryonic mouse brain inhibits GH and IGF-1 signaling post-birth, leading to growth retardation, small adult size, metabolic changes, and prolonged mean lifespan (Kappeler et al., 2008).
While further research is needed to fully elucidate the underlying regulatory mechanisms, existing studies in worms, flies, and mice clearly demonstrate the critical importance of neuroendocrine processes in the CNS for modulating the lifespan effects of IIS.
The Effects of Neuronal Stress-Sensing Pathways on Lifespan and Aging
The nervous system is not only a sensory organ, perceiving a wide range of environmental stressors, but also an integrator of this information, converting it into appropriate physiological and behavioral adaptations. We will examine two examples of stress-sensing pathways and their potential impacts on lifespan.
Exposure to acute stress, such as heat, heavy metals, or toxins, can induce proteotoxicity due to protein misfolding (reviewed in Åkerfelt et al., 2010). To counteract these insults, animals activate the heat shock response, mediated by heat shock transcription factor 1 (HSF-1) (Hsu et al., 2003; Morley and Morimoto, 2004; Cohen et al., 2006). Kourtis et al. (2012) demonstrated that HSF-1 is necessary for protecting against cytotoxicity induced by thermal or other stresses through the activation of small heat shock protein HSP-16.1. This protective mechanism, which also guards against neurodegeneration, is evolutionarily conserved (Kourtis et al., 2012). Given that thermosensory neurons and their circuits can non-autonomously regulate heat shock responses in C. elegans (Prahlad et al., 2008; Prahlad and Morimoto, 2011), sensory regulation of the HSF-1/HSP-16.1 response is likely also conserved.
Interestingly, HSF-1 activity promotes longevity even in the absence of acute stress (Hsu et al., 2003; Morley and Morimoto, 2004). Protein misfolding, whether polyglutamine repeat-mediated (Morley et al., 2002; van Ham et al., 2010) or not (David et al., 2010), increases with age. This suggests that protein aggregation is an inherent aspect of aging, not limited to disease-associated proteins (David et al., 2010). Since HSF-1 promotes protein disaggregation (Cohen et al., 2006), its activity in multiple tissues can affect lifespan even without acute stress (Hsu et al., 2003; Morley and Morimoto, 2004).
Animals also possess sensors for various gases crucial for physiological processes. Oxygen levels, for example, are sensed by specific soluble guanylyl cyclases (sGCs) in sensory neurons of C. elegans and Drosophila (Cheung et al., 2005; Chang et al., 2006; Rogers et al., 2006; Vermehren-Schmaedick et al., 2010). These sGCs regulate aerotactic behaviors: C. elegans prefers 7–11% oxygen and avoids hypoxic (2%) and hyperoxic (>14% O2) environments (Cheung et al., 2005; Chang et al., 2006; Rogers et al., 2006), while Drosophila larvae prefer a narrower range (∼21% O2) (Vermehren-Schmaedick et al., 2010). Hyperoxia avoidance in C. elegans also involves pain-sensing neurons and neurons integrating food availability and population density (Chang et al., 2006; Rogers et al., 2006). These sensory neurons enable rapid behavioral responses to ambient O2, allowing animals to migrate to optimal O2 environments for survival. Currently, sGCs are not known to affect lifespan, unlike receptor guanylyl cyclases, some of which inhibit longevity (Murphy et al., 2003; Alcedo and Kenyon, 2004).
Many cells also respond to O2, more slowly, through hypoxia-inducible transcription factor HIF-1, modifying O2-sensing neurons and neural circuits (Chang and Bargmann, 2008; Pocock and Hobert, 2010). Hypoxic HIF-1 activation shifts oxygen preferences and eliminates dependence on neurons integrating food and population density in O2-dependent responses (Chang and Bargmann, 2008). This HIF-1 effect requires coordinated action in neuronal and gonadal cells (Chang and Bargmann, 2008), whose outputs influence lifespan (Apfeld and Kenyon, 1999; Hsin and Kenyon, 1999; Wolkow et al., 2000; Broughton et al., 2005; Flatt et al., 2008).
The HIF-1 pathway influences C. elegans lifespan, with effects depending on environmental context (Chen et al., 2009; Mehta et al., 2009; Zhang et al., 2009; Lee et al., 2010; Leiser et al., 2011). hif-1 loss can extend lifespan at higher temperatures (25°C) (Chen et al., 2009; Leiser et al., 2011) or shorten it at lower temperatures (20°C) (Mehta et al., 2009; Lee et al., 2010). Since O2 perception is modulated by food-derived information (Chang et al., 2006; Rogers et al., 2006; Chang and Bargmann, 2008; Pocock and Hobert, 2010), temperature-dependent HIF-1 effects may reflect differences in bacterial food sources at 25°C versus 20°C. Consistent with this, HIF-1 interacts with the food-dependent TOR pathway in affecting lifespan (Chen et al., 2009). Similarly, population density affects O2-sensing (Chang et al., 2006; Rogers et al., 2006), and hif-1 lifespan effects observed by Zhang et al. (2009) might reflect higher animal density in their assays. Thus, HIF-1 function illustrates how environmental context and perception modulate a signaling pathway’s lifespan effects.
The Role of Mitochondria and the Electron Transport Chain in Brain Aging and Longevity
Mitochondria are critical cellular organelles that significantly contribute to the aging process, primarily through respiratory chain dysfunction, alterations in redox status, and the generation of reactive oxygen species (ROS) (Humphries et al., 2006; Mattson, 2006). The nervous system, with its high energy demands for processes like ion homeostasis, neurotransmission, and action potential firing, exhibits particularly active mitochondrial metabolism. The electron transport chain (ETC), located within the mitochondria, is the central machinery for cellular respiration and ATP production. Understanding its inputs and outputs is crucial to grasping mitochondrial function in aging.
Electron Transport Chain: Inputs and Outputs
The ETC’s primary function is to generate a proton gradient across the inner mitochondrial membrane, which is then used to drive ATP synthesis. Here are the key inputs and outputs:
Inputs:
- NADH and FADH2: These are electron carriers generated from the breakdown of glucose, fatty acids, and amino acids during cellular metabolism (e.g., glycolysis, Krebs cycle, beta-oxidation). They donate electrons to the ETC.
- Oxygen (O2): Oxygen acts as the final electron acceptor in the ETC. It is reduced to water (H2O).
- ADP and Inorganic Phosphate (Pi): These are the substrates for ATP synthase, the enzyme that uses the proton gradient to synthesize ATP.
Outputs:
- ATP (Adenosine Triphosphate): The primary energy currency of the cell. The ETC is essential for generating the majority of ATP in most cells.
- Water (H2O): A byproduct of oxygen reduction at the end of the ETC.
- Heat: The ETC is not perfectly efficient, and some energy is released as heat.
- Reactive Oxygen Species (ROS): Incomplete electron transfer in the ETC can lead to the generation of ROS, such as superoxide radicals. While mitochondria have antioxidant defenses, excessive ROS production can contribute to oxidative stress and damage.
In mammals, structural impairments in mitochondrial DNA and age-related decline in brain mitochondrial function are linked to age-dependent decreases in cognitive function and neuromuscular coordination (reviewed in Bishop et al., 2010; Escames et al., 2010; Chakrabarti et al., 2011; Yin et al., 2012). Mitochondrial dysfunction is also implicated in neurodegenerative diseases (reviewed in Eckert et al., 2011; Reddy and Reddy, 2011; Swerdlow, 2011; Troulinaki and Bano, 2012; Yin et al., 2012), although the distinction between functional changes in healthy brain aging and pathological processes in neurodegenerative diseases remains unclear. Current evidence suggests that neuronal mitochondria, and by extension the efficient operation of the electron transport chain, are vital for maintaining organismal homeostasis and influencing aging. Disruptions in Electron Transport Chain Inputs And Outputs can have cascading effects on neuronal function and longevity.
Several observations support the importance of proper neuronal mitochondrial function for lifespan and healthy aging. Expression of human mitochondrial UCPs, which can uncouple mitochondrial respiration from ATP synthesis, in adult fly neurons extends lifespan (Fridell et al., 2005, 2009; Humphrey et al., 2009). This effect likely occurs through reduced ILP secretion (Fridell et al., 2009; Humphrey et al., 2009), as human UCP2 regulates insulin secretion (Zhang et al., 2001). Interestingly, while moderate neuronal UCP expression lengthens lifespan (Fridell et al., 2005; Humphrey et al., 2009), high levels have the opposite effect (Humphrey et al., 2009; Figure 2). This mirrors studies showing that mild mitochondrial function reduction can extend lifespan, while severe impairment shortens it (Rea et al., 2007). Hypothetically, mild mitochondrial dysfunction might cause: (1) changes in ROS production levels, such as a decrease preserving DNA and protein structures, or a mild increase triggering compensatory mechanisms, or (2) changes in ROS types produced, stimulating longevity-promoting gene expression. These data suggest that increased lifespan associated with mild neuronal mitochondrial dysfunction (Dillin et al., 2002a; Rea and Johnson, 2003; Morrow et al., 2004; Fridell et al., 2005, 2009; Conti et al., 2006; Rea et al., 2007; Copeland et al., 2009; Humphrey et al., 2009; Lee et al., 2010; Figure 2) represents a compensatory mechanism maintaining homeostasis. Disruptions in the electron transport chain, affecting both inputs like NADH availability and outputs like ATP production and ROS generation, are likely central to these phenomena.
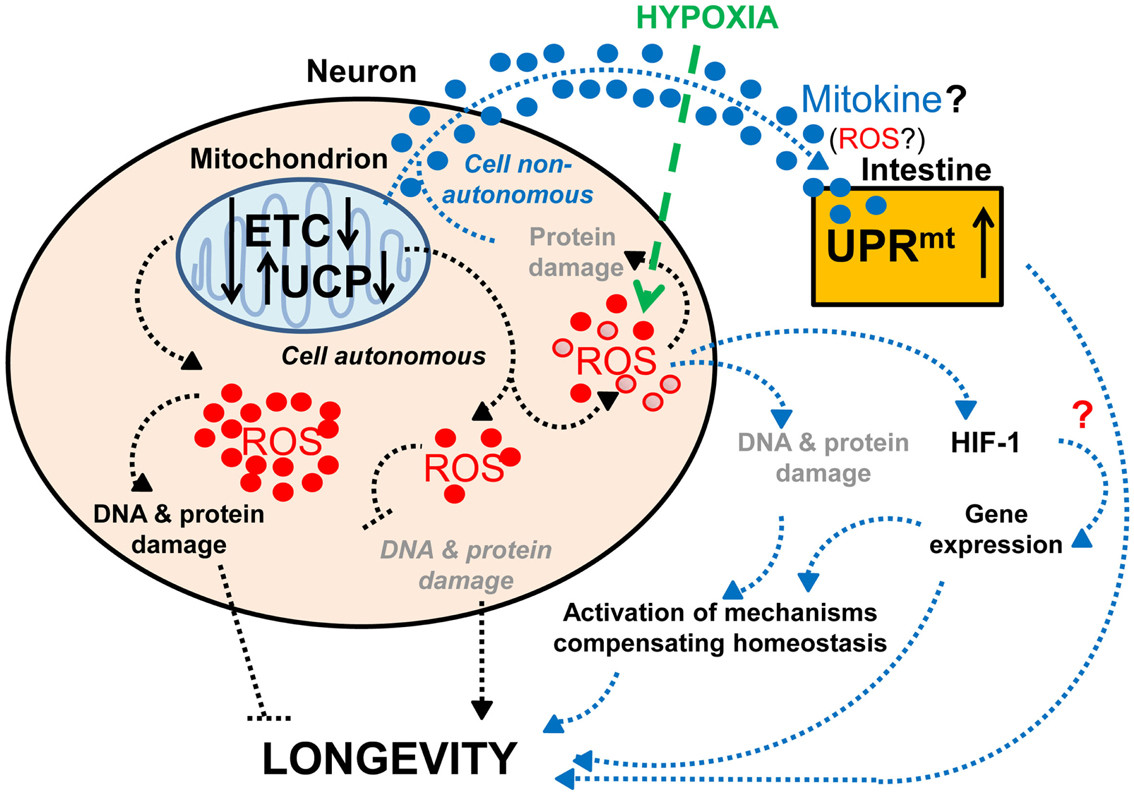 Effects of neuronal mitochondrial UCP and the electron transport chain on longevity
Effects of neuronal mitochondrial UCP and the electron transport chain on longevity
Figure 2. Mitochondrial function and longevity. This figure illustrates the effects of neuronal mitochondrial UCP and the electron transport chain on longevity. Lifespan is modulated by altered mitochondrial function in neurons: a lower level of UCP and electron transport chain (ETC) expression lengthens lifespan, whereas a higher level of UCP and ETC expression has the opposite effect on lifespan (Fridell et al., 2005; Rea et al., 2007; Copeland et al., 2009; Humphrey et al., 2009; Durieux et al., 2011). The lifespan increase observed with mild mitochondrial dysfunction may hypothetically be due to (1) a decrease in ROS production and DNA and protein damage (denoted in gray and italics) or (2) a mild increase in ROS production and DNA and protein damage (denoted in gray), which can activate compensatory mechanisms. Alternatively, mitochondria-dependent lifespan increases might also be due to other compensatory mechanisms induced by a change in the types of ROS produced (red • versus red ^). Neuronal mitochondrial dysfunction can also induce a cell non-autonomous UPRmt in intestinal cells and lead to lifespan extension, via a proposed mitokine, like ROS (Durieux et al., 2011). However, intestinal UPRmt response is necessary but not sufficient to promote longevity (Durieux et al., 2011). Since HIF-1 activates survival genes in response to hypoxia and a mild inhibition of mitochondrial ETC, which involves an increase in ROS levels (Lee et al., 2010), the possible role of HIF-1 in this process is speculated (denoted by a red “?”).
Reduced mitochondrial respiratory chain function in the nervous system also induces a mitochondria-specific unfolded protein response (UPRmt) in intestinal cells, extending lifespan (Durieux et al., 2011; Figure 2). Interestingly, similar mitochondrial impairment in muscle cells also induces UPRmt, but without lifespan extension (Durieux et al., 2011), suggesting UPRmt alone is insufficient for longevity. However, UPRmt induction is necessary for the long-life phenotype from reduced mitochondrial respiration (Durieux et al., 2011). These findings suggest neuronal mitochondrial dysfunction extends lifespan by producing an unknown signal, acting with the UPRmt-inducing signal. While this signal remains unidentified, HIF-1 pathway involvement is a tempting speculation. HIF-1 modifies neuronal activities (Chang and Bargmann, 2008; Pocock and Hobert, 2010) and promotes longevity in response to mild mitochondrial respiration inhibition via increased ROS levels (Lee et al., 2010). While longevity-promoting effects of increased ROS (Lee et al., 2010) contradict the hypothesis of ROS shortening lifespan through oxidative damage (Humphries et al., 2006; Mattson, 2006), it aligns with mitohormesis, where higher ROS leads to increased stress resistance (Schulz et al., 2007). Alternatively, specific ROS types might act as signaling molecules activating survival pathways (Bishop et al., 2010; Lee et al., 2010; Durieux et al., 2011).
As the major ROS source, mitochondria are deeply involved in pathway crosstalk. Mitochondrial activity is regulated by major longevity pathways, including IIS, TOR, and JNK signaling (reviewed in Troulinaki and Bano, 2012). ROS-mediated JNK activity induction (Wang et al., 2005), leading to JNK translocation from cytoplasm to mitochondria, is proposed as fundamental in transducing cytosolic signals to mitochondria in the aging mammalian brain (Schroeter et al., 2003; Eminel et al., 2004; Zhou et al., 2008, 2009).
ROS signaling also modulates mitochondrial homeostasis, involving constant organelle remodeling via fusion, fission, and autophagy (reviewed in Lemasters, 2005; Lee et al., 2012; Palikaras and Tavernarakis, 2012; Liesa and Shirihai, 2013). This regulated remodeling appears adaptive to cell energy expenditure and demands (reviewed in Liesa and Shirihai, 2013). Mitochondrial fusion and fission distribute damaged components across the network, while mitophagy removes highly damaged mitochondria (reviewed in Lemasters, 2005; Lee et al., 2012; Palikaras and Tavernarakis, 2012). Increased ROS levels can shift the fusion/fission balance toward mitophagy (reviewed in Lemasters, 2005; Lee et al., 2012; Palikaras and Tavernarakis, 2012). Mitophagy requires genes implicated in Parkinson’s disease, such as PINK1 and Parkin. PINK1 senses damaged mitochondria and recruits Parkin to induce mitophagy (Narendra et al., 2008, 2010). Dysregulation of mitochondrial remodeling, including mitophagy, due to excess ROS, likely contributes to age-associated neurodegenerative diseases (reviewed in Batlevi and La Spada, 2011; Palikaras and Tavernarakis, 2012). Maintaining the balance of electron transport chain inputs and outputs and overall mitochondrial health is therefore crucial for neuronal longevity.
Feedback, Compensatory, and Feed-Forward Mechanisms in Longevity-Modulating Pathways
The studies discussed highlight the presence of significant feedback mechanisms within the nervous system. Feedback loops are vital for regulating physiology and metabolism, especially in maintaining homeostasis, often controlled by hormones (reviewed in Baker and Thummel, 2007; Leopold and Perrimon, 2007; Fielenbach and Antebi, 2008; Rajan and Perrimon, 2011; Hill et al., 2012). Many endocrine feedback mechanisms are thought to modulate aging and lifespan (Tatar et al., 2003; Murphy et al., 2007; Fielenbach and Antebi, 2008; Broughton and Partridge, 2009; Karpac and Jasper, 2009; Karpac et al., 2009; Tazearslan et al., 2009; Landis and Murphy, 2010), with the nervous system implicated in several (Hwangbo et al., 2004; Broughton et al., 2008; Flatt et al., 2008; Grönke et al., 2010; Alic et al., 2011; Boulias and Horvitz, 2012). We focus on IIS-related feedback mechanisms involving the nervous system.
One example is adipose tissue-brain communication via IIS. Hwangbo et al. (2004) found that D. melanogaster FOXO overexpression in head fat body (mammalian liver/adipose equivalent) extends lifespan and reduces ILP2 levels produced in CNS IPCs. This suggests lifespan extension is caused by FOXO-mediated negative feedback regulation of neural ILP production. This aligns with IPC ablation extending lifespan (Wessells et al., 2004; Broughton et al., 2005), likely due to lowered ILP2, ILP3, and ILP5 levels (Broughton et al., 2008; Grönke et al., 2010). Interestingly, a fat body-produced humoral factor remotely controls insulin secretion from IPCs (Geminard et al., 2009; Tatar, 2009), but whether this factor modulates lifespan is unknown.
Another example is endocrine communication between gonad and brain. Similar to C. elegans findings (Hsin and Kenyon, 1999; Arantes-Oliveira et al., 2002), Flatt et al. (2008) found that germline stem cell (GSC) ablation extends Drosophila lifespan. Despite peripheral tissue IIS impairment, fly GSC ablation upregulates ILP2, ILP3, and ILP5 production in brain IPCs (Flatt et al., 2008). Since neurally produced ILPs bind to the insulin-like receptor (InR) on GSCs to regulate GSC proliferation in the gonad (LaFever and Drummond-Barbosa, 2005; Hsu et al., 2008), GSCs in the gonad may exert negative feedback on brain ILP production. The signaling molecule remains unknown, but IMP-L2, an insulin-binding protein, is a promising candidate. IMP-L2, expressed in the germline niche (Terry et al., 2006), limits free ILP availability by sequestering them from InR, antagonizing systemic IIS (Honegger et al., 2008). This protein is upregulated in germline-less, long-lived flies with ILP overproduction (Flatt et al., 2008). Similar to germline-less flies, IMP-L2 upregulation extends lifespan and increases ILP2, ILP3, and ILP5 levels, while ilp2, ilp3, and ilp5 deletion decreases IMP-L2 (Grönke et al., 2010; Alic et al., 2011). These observations support IMP-L2 as part of a gonad-brain signaling circuit regulating neural ILP levels (Figure 3).
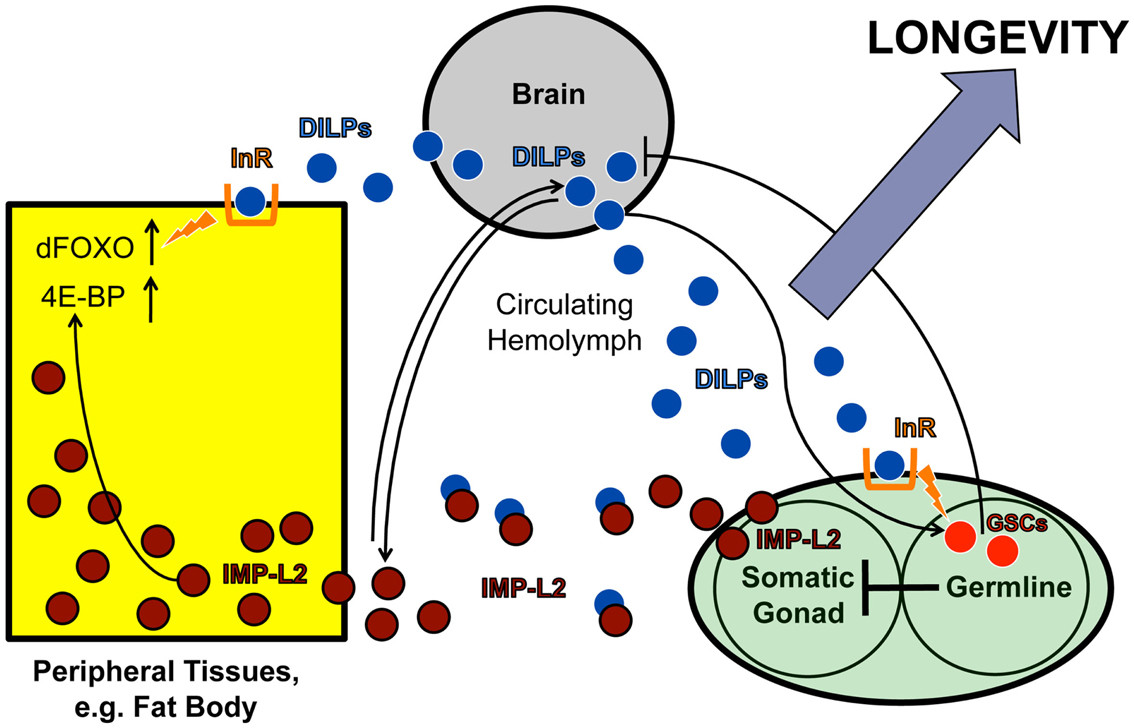 IMP-L2-mediated endocrine feedback loop between brain and ovary
IMP-L2-mediated endocrine feedback loop between brain and ovary
Figure 3. Brain-ovary feedback loop. This model illustrates the IMP-L2-mediated endocrine feedback loop between brain and ovary, based on findings in LaFever and Drummond-Barbosa (2005), Flatt et al. (2008), and Alic et al. (2011). ILPs produced in the brain bind to the ovarian InR and stimulate GSC proliferation. GSC proliferation likely downregulates ILP production in IPCs since GSC ablation increases ILP transcription, suggesting a negative feedback loop between brain and ovarian tissues. This feedback loop might be partly mediated by IMP-L2, an ILP-binding protein inhibiting insulin signaling. GSC ablation strongly upregulates IMP-L2. GSC ablation and IMP-L2 overexpression cause similar phenotypes: increased lifespan, ilp2, ilp3, and ilp5 upregulation, and increased DAF-16/FOXO target expression (e.g., 4E-BP), although other DAF-16/FOXO activity aspects remain unaltered. This suggests IMP-L2 mediates the long-lifespan phenotype of GSC-ablated flies by modulating insulin signaling.
While detailed physiological consequences, especially for aging and longevity, are often unknown, transcriptional regulation also involves feedback mechanisms. For example, some of the seven Drosophila ILPs exhibit feedback regulation (Figure 4A): IPC-expressed ilp3 is needed for normal ilp2 and ilp5 expression in IPCs, while ilp2 knockdown upregulates ilp3 and ilp5 expression in IPCs (Broughton et al., 2008; Grönke et al., 2010). Similar feedback loops exist for other IIS components: Drosophila FOXO (dFOXO), activated when InR signaling is downregulated, activates InR transcription (Puig et al., 2003; Puig and Tjian, 2005).
Intriguingly, besides feedback loops, aging genetic studies are revealing compensatory and feed-forward regulatory mechanisms. For example, fat body-specific ilp6 upregulation seems to compensate for brain-specific ilp2, ilp3, and ilp5 loss (Figure 4B; Grönke et al., 2010). Like Drosophila, C. elegans exhibits feed-forward regulation between ILPs (Murphy et al., 2007). Increased DAF-16/FOXO activity in one tissue increases DAF-16/FOXO activity in other tissues via feed-forward regulation, requiring ins-7 ILP expression inhibition in the C. elegans intestine (Figure 4C; Murphy et al., 2003, 2007). These studies exemplify the complexity of feedback, compensatory, and feed-forward mechanisms relevant to modulating aging and longevity.
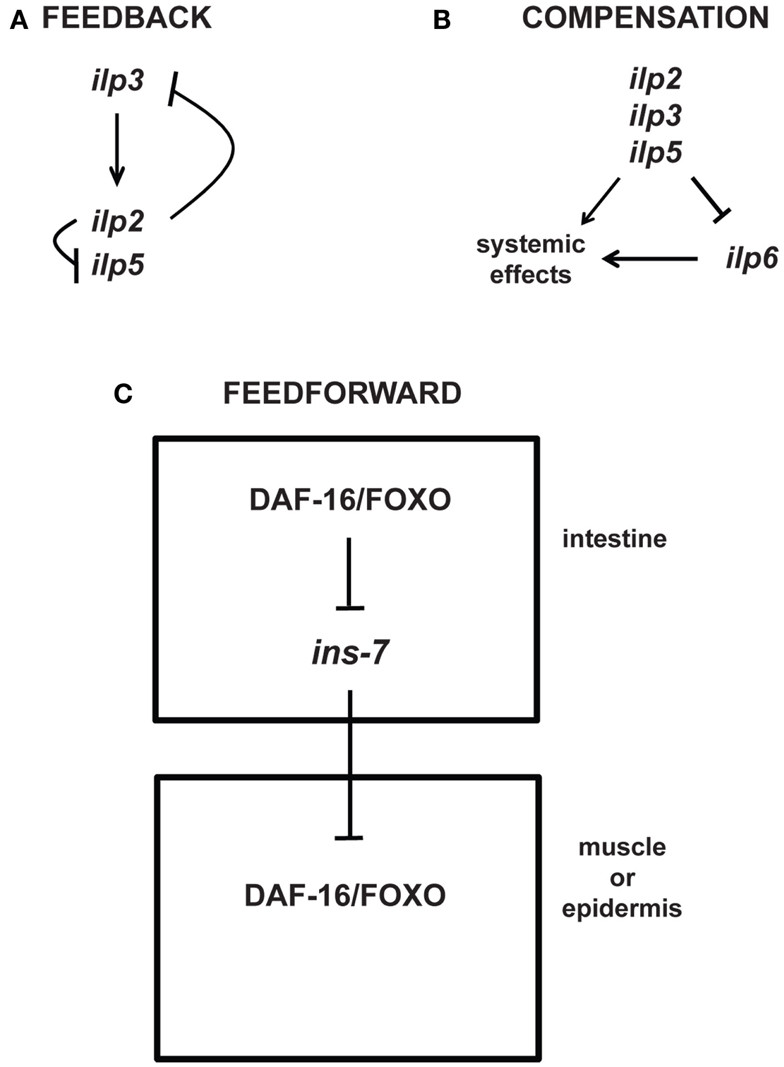 Feedback, compensatory, and feed-forward mechanisms in the longevity-modulating insulin signaling pathway
Feedback, compensatory, and feed-forward mechanisms in the longevity-modulating insulin signaling pathway
Figure 4. Regulatory mechanisms in insulin signaling. This figure depicts feedback, compensatory, and feed-forward mechanisms in the longevity-modulating insulin signaling pathway. (A) Neuronally produced Drosophila insulin-like peptides exhibit feedback regulation among each other (Broughton et al., 2008; Grönke et al., 2010). (B) The systemic activities of Drosophila neuronal ilp2, ilp3, and ilp5 can be compensated by the systemic activity of ilp6 produced from the head fat body (Grönke et al., 2010). (C) C. elegans ILP signaling between tissues (i.e., intestine to muscle or epidermis) involves feed-forward regulation via transcriptional inhibition of the ILP ins-7 (Murphy et al., 2007).
Temporal Requirements of Longevity-Influencing Genes
Current data suggests adult-expressed neuronal genes significantly affect aging and longevity (reviewed in Broughton and Partridge, 2009). However, the extent to which genes regulating nervous system development and circuitry influence homeostasis and longevity remains unclear. Interestingly, early-life environmental influences may have “carry-over” effects into adulthood, impacting lifespan (Gavrilov and Gavrilova, 2011; Saino et al., 2012). For example, early-age biomarkers affecting gene activity and chromosome structure can predict life expectancy (Baeriswyl et al., 2009; Pincus and Slack, 2010; Heidinger et al., 2012). Similarly, C. elegans age-related behaviors are associated with distinct transcriptomes, and aggregate gene expression profiles can predict age and health states (Golden et al., 2008). These data suggest genes involved in developmental canalization (or “robustness”) might have long-term effects on physiological homeostasis and somatic maintenance later in life. Canalization is predicted to be a generic feature of developmental gene networks (Siegal and Bergman, 2002; Flatt, 2005).
Pleiotropic gene action, where a gene’s developmental effect differs from its adult effect, is a plausible mechanism for these “carry-over” effects. The same gene variant might have pleiotropic roles in development versus lifespan (e.g., Dillin et al., 2002b). A gene can also have different lifespan effects depending on temporal activity, as seen with Drosophila p53 overexpression, which can have different lifespan effects in females and males depending on developmental versus adult expression (Waskar et al., 2009). Key lifespan modulators like mitochondrial electron transport chain components, microRNAs, HSF-1, and FOXO can have “carry-over” effects on adult lifespan when manipulated (overexpressed or silenced) during early larval development and/or early adulthood (Dillin et al., 2002a,b; Giannakou et al., 2007; Rea et al., 2007; Durieux et al., 2011; Pincus et al., 2011; Volovik et al., 2012). Age-dependent expression changes in neocortical genes, playing roles in both development and age-related cognitive decline and brain dysfunction, are other examples (reviewed in Huffman, 2012).
Pleiotropic genes demonstrate distinct functional roles in development versus aging, uncoupling their gene functions between these processes (Chen et al., 2007; Shen et al., 2009; Thyagarajan et al., 2010). Strong loss-of-function mutations can affect C. elegans embryonic development, while weaker alleles of the same gene affect adult lifespan (Kenyon et al., 1993; Kimura et al., 1997; Gems et al., 1998; Boehm and Slack, 2005), suggesting essential developmental genes can have late-life deleterious effects. Liu et al. (2012) showed miRNA signaling silences a set of these developmental genes in adulthood, neutralizing late-acting deleterious effects. miR-34-mediated silencing of steroid pathway gene E74A in adult Drosophila maintains brain integrity and viability (Liu et al., 2012).
Epigenetic modifications are another mechanism potentially involved in “carry-over” effects on lifespan and aging. Rodent experiments show experiences during sensitive brain development periods influence DNA methylation patterns, altering gene transcription throughout life and promoting specific phenotypic outcomes (Roth and Sweatt, 2011). The “heterochromatin loss model of aging” proposes heterochromatin domains established early in embryogenesis are gradually lost with age, resulting in aberrant and age-associated gene expression (Villeponteau, 1997). Genetic manipulation of HP1 levels and JAK/STAT signaling suggests heterochromatin formation prevents premature aging (Larson et al., 2012). These preliminary observations suggest epigenetic changes play a role in aging and lifespan.
Evolutionary Implications of Longevity-Modulating Neuronal Mechanisms
Classical evolutionary aging theory posits that aging mechanisms should differ across species (Williams, 1957; Reznick, 2005), but recent studies suggest conserved longevity pathways (reviewed in Partridge and Gems, 2002; Tatar et al., 2003; Kenyon, 2005; Partridge et al., 2005; Smith et al., 2008; Flatt and Schmidt, 2009; Fontana et al., 2010; Nakagawa et al., 2012; Wuttke et al., 2012). Lifespan varies orders of magnitude across species (Finch, 1990; Stearns, 1992; Nabholz et al., 2008; Li and de Magalhães, 2011), but molecular longevity mechanisms are mainly studied in short-lived model systems, potentially biasing our understanding (Deweerdt, 2012). While many conserved, pleiotropic signaling pathways implicated in aging have neuronal roles, not all directly impinge on aging. The evolutionary conservation of neuronal longevity mechanisms remains largely unclear.
A study comparing gene expression profiles during aging in mouse, rhesus macaque, and human brains indicates only a small subset of age-dependent expression changes are conserved (Loerch et al., 2008). These include neuroprotective apolipoprotein D (APOD), upregulated with age in all three species, with Drosophila homologs affecting lifespan (Ruiz et al., 2011). Calcium/calmodulin-dependent protein kinase IV (CAMK4), regulating synaptic plasticity (Ho et al., 2000), is downregulated with age in all three species (Loerch et al., 2008). However, most genes showed inconsistent age-dependent patterns across species, leading authors to conclude humans and rhesus macaques have diverged greatly from mice, evidenced by dramatic age-dependent repression of human and macaque neuronal genes (Loerch et al., 2008). While these results suggest neuronal aging and longevity mechanisms may not be highly conserved across taxa, Fonseca et al. (2005) provide a counter-example. Across arthropods with lifespans varying by three orders of magnitude, neuronal lipofuscin deposition is highly correlated with lifespan, suggesting age-dependent brain damage accumulation may drive senescence (Fonseca et al., 2005).
At the microevolutionary level, it’s unclear if natural lifespan variation is based on allelic variation within genes and pathways previously found to affect longevity in lab studies (Flatt, 2004; Paaby and Schmidt, 2008; Flatt and Schmidt, 2009). Some studies failed to confirm lifespan effects of natural candidate longevity gene variants (Geiger-Thornsberry and Mackay, 2004), while increasing evidence suggests genetic variation in candidate longevity genes contributes to lifespan and life history trait variation in natural populations (Schmidt et al., 2000; Paaby and Schmidt, 2008; Suh et al., 2008; Paaby et al., 2010; Rose et al., 2010, 2011; Pijpe et al., 2011; Luisi et al., 2012).
A striking example is FOXO3A, a human ortholog of Drosophila FOXO and C. elegans DAF-16. Independent studies of natural FOXO3A polymorphisms in Japanese, German, French, Italian, and Han Chinese populations found specific variants associated with exceptional longevity in human centenarians (Willcox et al., 2008; Anselmi et al., 2009; Flachsbart et al., 2009; Li et al., 2009; Pawlikowska et al., 2009; Soerensen et al., 2010; Zeng et al., 2010). Despite potential ascertainment bias, these results suggest FOXO plays a functional role in regulating lifespan in model organisms, and natural alleles can measurably affect lifespan. Similar associations between natural polymorphisms and human longevity exist for IGF-1R (Suh et al., 2008). Drosophila InR locus natural alleles also affect life history traits linked to longevity (Paaby et al., 2010).
A similar pattern emerges for natural variants of genes involved in neuronal lifespan regulation: correlations exist between longevity and genes functioning in (1) neuronal development (Rybina and Pasyukova, 2010; Walter et al., 2011), (2) neural circuitry (De Benedictis et al., 1998; De Luca et al., 2001, 2003; Carbone et al., 2006), or (3) neuronal tissue uncoupling processes (Rose et al., 2011).
Conclusions and Perspectives
This review has summarized recent knowledge of neuronal inputs and outputs affecting aging and longevity, primarily focusing on genetically tractable model organisms like flies, worms, and mice. Despite remaining unknowns, it is evident that neuronal activities profoundly influence aging and longevity. The nervous system, particularly the neuroendocrine system, is deeply involved in regulating animal physiology, including homeostasis and survival in response to environmental changes. This makes its role in aging physiologically and evolutionarily unsurprising. However, many puzzles remain. For instance, IIS downregulation can positively impact lifespan but also impair neuronal survival and CNS function in old age (Broughton and Partridge, 2009). These distinct IIS effects might depend on tissue- or temporal-specific pathway activities. Pleiotropic IIS effects highlight the need to better understand how, why, and when “brain aging” and “organismal aging” are coupled or decoupled. Understanding developmental “carry-over” effects on adult lifespan requires further insight into tissue-, age-, and stage-specificity of neuronal effects on aging and longevity. Our knowledge of intricate interactions in neuronal aging and longevity regulation is still rudimentary. Interactions between different “longevity” pathways in the brain, or cross-talk between tissues (gonad, adipose tissue) and the CNS in modulating whole-organism lifespan, remain largely unexplored. Despite recent progress, exciting discoveries lie ahead in elucidating neuronal aspects of aging and longevity.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Joy Alcedo has been supported by the Novartis Research Foundation, the Swiss National Science Foundation (SNF, 31003A_134958) and Wayne State University. Thomas Flatt acknowledges support from the Austrian Science Foundation (FWF, P21498-B11), the Swiss National Science Foundation (SNF, PP00P3_133641), and the Wissenschaftskolleg zu Berlin. Elena G. Pasyukova was supported by the Presidium of the Russian Academy of Sciences and the Russian Foundation for Basic Research (#12-04-01182-a).
References
Åkerfelt, M., Morimoto, R. I., and Sistonen, L. (2010). Heat shock factors: integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 11, 545–555.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Alcedo, J., and Kenyon, C. (2004). Regulation of C. elegans longevity by specific gustatory and olfactory neurons. Neuron 41, 45–55.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Alcedo, J., Maier, W., and Ch’ng, Q. (2010). “Sensory influence on homeostasis and lifespan: molecules and circuits,” in Protein Metabolism and Homeostasis in Aging, ed. N. Tavernarakis (Austin, TX: Landes Bioscience), 197–210.
Alic, N., Hoddinott, M. P., Vinti, G., and Partridge, L. (2011). Lifespan extension by increased expression of the Drosophila homologue of the IGFBP7 tumour suppressor. Aging Cell 10, 137–147.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Anselmi, C. V., Malovini, A., Roncarati, R., Novelli, V., Villa, F., Condorelli, G., et al. (2009). Association of the FOXO3A locus with extreme longevity in a southern Italian centenarian study. Rejuv. Res. 12, 95–104.
Apfeld, J., and Kenyon, C. (1999). Regulation of lifespan by sensory perception in Caenorhabditis elegans. Nature 402, 804–809.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Arantes-Oliveira, N., Apfeld, J., Dillin, A., and Kenyon, C. (2002). Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science 295, 502–505.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Baeriswyl, S., Diard, M., Mosser, T., Leroy, M., Manière, X., Taddei, F., et al. (2009). Modulation of aging profiles in isogenic populations of Caenorhabditis elegans by bacteria causing different extrinsic mortality rates. Biogerontology 11, 53–65.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Baker, K. D., and Thummel, C. S. (2007). Diabetic larvae and obese flies – emerging studies of metabolism in Drosophila. Cell Metab. 6, 257–266.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Bargmann, C. I. (1998). Neurobiology of the Caenorhabditis elegans genome. Science 282, 2028–2033.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Bargmann, C. I., and Horvitz, H. R. (1991). Control of larval development by chemosensory neurons in Caenorhabditis elegans. Science 251, 1243–1246.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Bateman, J. M., and McNeill, H. (2006). Insulin/IGF signalling in neurogenesis. Cell. Mol. Life Sci. 63, 1701–1705.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Batlevi, Y., and La Spada, A. R. (2011). Mitochondrial autophagy in neural function, neurodegenerative disease, neuron cell death, and aging. Neurobiol. Dis. 43, 46–51.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Bauer, J. H., Chang, C., Morris, S. N., Hozier, S., Andersen, S., Waitzman, J. S., et al. (2007). Expression of dominant-negative Dmp53 in the adult fly brain inhibits insulin signaling. Proc. Natl. Acad. Sci. U.S.A. 104, 13355–13360.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Berryman, D. E., Christiansen, J. S., Johannsson, G., Thorner, M. O., and Kopchick, J. J. (2008). Role of the GH/IGF-1 axis in lifespan and healthspan: lessons from animal models. Growth Horm. IGF Res. 18, 455–471.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Bishop, N. A., and Guarente, L. (2007). Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature 447, 545–549.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Bishop, N. A., Lu, T., and Yankner, B. A. (2010). Neural mechanisms of ageing and cognitive decline. Nature 464, 529–535.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Boehm, M., and Slack, F. (2005). A developmental timing microRNA and its target regulate life span in C. elegans. Science 310, 1954–1957.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Boulias, K., and Horvitz, H. R. (2012). The C. elegans microRNA mir-71 acts in neurons to promote germline-mediated longevity through regulation of DAF-16/FOXO. Cell Metab. 15, 439–450.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Broughton, S., Alic, N., Slack, C., Bass, T., Ikeya, T., Vinti, G., et al. (2008). Reduction of DILP2 in Drosophila triages a metabolic phenotype from lifespan revealing redundancy and compensation among DILPs. PLoS ONE 3:e3721. doi:10.1371/journal.pone.0003721
Broughton, S., and Partridge, L. (2009). Insulin/IGF-like signalling, the central nervous system and aging. Biochem. J. 418, 1–12.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Broughton, S. J., Piper, M. D. W., Ikeya, T., Bass, T. M., Jacobsen, J., Driege, Y., et al. (2005). Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc. Natl. Acad. Sci. U.S.A. 102, 3105–3110.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Carbone, M. A., Jordan, K. W., Lyman, R. F., Harbison, S. T., Leips, J., Morgan, T. J., et al. (2006). Phenotypic variation and natural selection at catsup, a pleiotropic quantitative trait gene in Drosophila. Curr. Biol. 16, 912–919.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Chakrabarti, S., Munshi, S., Kalpita Banerjee, R., Ishita Guha Thakurta, I. G., Sinha, M., and Bagh, M. B. (2011). Mitochondrial dysfunction during brain aging: role of oxidative stress and modulation by antioxidant supplementation. Aging Dis. 2, 242–256.
Pubmed Abstract | Pubmed Full Text
Challet, E., Caldelas, I., Graff, C., and Pévet, P. (2003). Synchronization of the molecular clockwork by light- and food-related cues in mammals. Biol. Chem. 384, 711–719.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Chang, A. J., and Bargmann, C. I. (2008). Hypoxia and the HIF-1 transcriptional pathway reorganize a neuronal circuit for oxygen-dependent behavior in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 105, 7321–7326.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Chang, A. J., Chronis, N., Karow, D. S., Marletta, M. A., and Bargmann, C. I. (2006). A distributed chemosensory circuit for oxygen preference in C. elegans. PLoS Biol. 4:e274. doi:10.1371/journal.pbio.0040274
Chen, D., Pan, K. Z., Palter, J. E., and Kapahi, P. (2007). Longevity determined by developmental arrest genes in Caenorhabditis elegans. Aging Cell 6, 525–533.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Chen, D., Thomas, E. L., and Kapahi, P. (2009). HIF-1 modulates dietary restriction-mediated lifespan extension via IRE-1 in Caenorhabditis elegans. PLoS Genet. 5:e1000486. doi:10.1371/journal.pgen.1000486
Chen, Z., Hendricks, M., Cornils, A., Maier, W., Alcedo, J., and Zhang, Y. (2013). Two insulin-like peptides antagonistically regulate aversive olfactory learning in C. elegans. Neuron 77, 572–585.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Cheung, B. H. H., Cohen, M., Rogers, C., Albayram, O., and de Bono, M. (2005). Experience-dependent modulation of C. elegans behavior by ambient oxygen. Curr. Biol. 15, 905–917.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Chrysis, D., Calikoglu, A. S., Ye, P., and D’Ercole, A. J. (2001). Insulin-like growth factor-I overexpression attenuates cerebellar apoptosis by altering the expression of Bcl family proteins in a developmentally specific manner. J. Neurosci. 21, 1481–1489.
Pubmed Abstract | Pubmed Full Text
Cohen, E., Bieschke, J., Perciavalle, R. M., Kelly, J. W., and Dillin, A. (2006). Opposing activities protect against age-onset proteotoxicity. Science 313, 1604–1610.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Conti, B., Sanchez-Alavez, M., Winsky-Sommerer, R., Morale, M. C., Lucero, J., Brownell, S., et al. (2006). Transgenic mice with a reduced core body temperature have an increased life span. Science 314, 825–828.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Copeland, J. M., Cho, J., Lo, T., Hur, J. H., Bahadorani, S., Arabyan, T., et al. (2009). Extension of Drosophila life span by RNAi of the mitochondrial respiratory chain. Curr. Biol. 19, 1591–1598.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Cornils, A., Gloeck, M., Chen, Z., Zhang, Y., and Alcedo, J. (2011). Specific insulin-like peptides encode sensory information to regulate distinct developmental processes. Development 138, 1183–1193.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
David, D. C., Ollikainen, N., Trinidad, J. C., Cary, M. P., Burlingame, A. L., and Kenyon, C. (2010). Widespread protein aggregation as an inherent part of aging in C. elegans. PLoS Biol. 8:e1000450. doi:10.1371/journal.pbio.1000450
De Benedictis, G., Carotenuto, L., Carrieri, G., De Luca, M., Falcone, E., Rose, G., et al. (1998). Gene/longevity association studies at four autosomal loci (REN, THO, PARP, SOD2). Eur. J. Hum. Genet. 6, 534–541.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
De Luca, M., Rose, G., Bonafè, M., Garasto, S., Greco, V., Weir, B. S., et al. (2001). Sex-specific longevity associations defined by tyrosine hydroxylase-insulin-insulin growth factor 2 haplotypes on the 11p15.5 chromosomal region. Exp. Gerontol. 36, 1663–1671.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
De Luca, M., Roshina, N. V., Geiger-Thornsberry, G. L., Lyman, R. F., Pasyukova, E. G., and Mackay, T. F. C. (2003). Dopa decarboxylase (Ddc) affects variation in Drosophila longevity. Nat. Genet. 34, 429–433.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Deweerdt, S. (2012). Comparative biology: looking for a master switch. Nature 492, S10–S11.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Dillin, A., Hsu, A. L., Arantes-Oliveira, N., Lehrer-Graiwer, J., Hsin, H., Fraser, A. G., et al. (2002a). Rates of behavior and aging specified by mitochondrial function during development. Science 298, 2398–2401.
Dillin, A., Crawford, D. K., and Kenyon, C. (2002b). Timing requirements for insulin/IGF-1 signaling in C. elegans. Science 298, 830–834.
Durieux, J., Wolff, S., and Dillin, A. (2011). The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell 144, 79–91.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Eckert, A., Schmitt, K., and Götz, J. (2011). Mitochondrial dysfunction – the beginning of the end in Alzheimer’s disease? Separate and synergistic modes of tau and amyloid-β toxicity. Alzheimers Res. Ther. 3, 15. doi:10.1186/alzrt74
Eminel, S., Klettner, A., Roemer, L., Herdegen, T., and Waetzig, V. (2004). JNK2 translocates to the mitochondria and mediates cytochrome c release in PC12 cells in response to 6-hydroxydopamine. J. Biol. Chem. 279, 55385–55392.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Enell, L. E., Kapan, N., Söderberg, J. A. E., Kahsai, L., and Nässel, D. R. (2010). Insulin signaling, lifespan and stress resistance are modulated by metabotropic GABA receptors on insulin producing cells in the brain of Drosophila. PLoS ONE 5:e15780. doi:10.1371/journal.pone.0015780
Escames, G., López, A., García, J. A., García, L., Acuña-Castroviejo, D., García, J. J., et al. (2010). The role of mitochondria in brain aging and the effects of melatonin. Curr. Neuropharmacol. 8, 182–193.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Fielenbach, N., and Antebi, A. (2008). C. elegans dauer formation and the molecular basis of plasticity. Genes Dev. 22, 2149–2165.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Finch, C. E. (1990). Longevity, Senescence, and the Genome. Chicago: The University of Chicago Press.
Flachsbart, F., Caliebe, A., Kleindorp, R., Blanché, H., von Eller-Eberstein, H., Nikolaus, S., et al. (2009). Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc. Natl. Acad. Sci. U.S.A. 106, 2700–2705.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Flatt, T. (2004). Assessing natural variation in genes affecting Drosophila lifespan. Mech. Ageing Dev. 125, 155–159.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Flatt, T. (2005). The evolutionary genetics of canalization. Q. Rev. Biol. 80, 287–316.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Flatt, T., Min, K.-J., D’Alterio, C., Villa-Cuesta, E., Cumbers, J., Lehmann, R., et al. (2008). Drosophila germ-line modulation of insulin signaling and lifespan. Proc. Natl. Acad. Sci. U.S.A. 105, 6368–6373.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Flatt, T., and Schmidt, P. S. (2009). Integrating evolutionary and molecular genetics of aging. Biochim. Biophys. Acta 1790, 951–962.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Fonseca, D. B., Brancato, C. L., Prior, A. E., Shelton, P. M., and Sheehy, M. R. (2005). Death rates reflect accumulating brain damage in arthropods. Proc. Biol. Sci. 272, 1941–1947.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Fontana, L., Partridge, L., and Longo, V. D. (2010). Extending healthy life span – from yeast to humans. Science 328, 321–328.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Fridell, Y. W., Hoh, M., Kréneisz, O., Hosier, S., Chang, C., Scantling, D., et al. (2009). Increased uncoupling protein (UCP) activity in Drosophila insulin-producing neurons attenuates insulin signaling and extends lifespan. Aging (Albany, NY) 1, 699–713.
Fridell, Y. W., Sanchez_Blanco, A., Silvia, B. A., and Helfand, S. L. (2005). Targeted expression of the human uncoupling protein 2 (hUCP2) to adult neurons extends life span in the fly. Cell Metab. 1, 145–152.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Gaglia, M. M., Jeong, D.-E., Ryu, E.-A., Lee, D., Kenyon, C., and Lee, S.-J. (2012). Genes that act downstream of sensory neurons to influence longevity, dauer formation, and pathogen responses in Caenorhabditis elegans. PLoS Genet. 8:e1003133. doi:10.1371/journal.pgen.1003133
Gavrilov, L. A., and Gavrilova, N. S. (2011). Season of birth and exceptional longevity: comparative study of American centenarians, their siblings, and spouses. J. Aging Res. 2011:104616. doi:10.4061/2011/104616
Geiger-Thornsberry, G. L., and Mackay, T. F. C. (2004). Quantitative trait loci affecting natural variation in Drosophila longevity. Mech. Ageing Dev. 125, 179–189.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Geminard, C., Rulifson, E. J., and Leopold, P. (2009). Remote control of insulin secretion by fat cells in Drosophila. Cell Metab. 10, 199–207.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Gems, D., Sutton, A. J., Sundermeyer, M. L., Albert, P. S., King, K. V., Edgley, M. L., et al. (1998). Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics 150, 129–155.
Pubmed Abstract | Pubmed Full Text
Giannakou, M. E., Goss, M., Jacobson, J., Vinti, G., Leevers, S. J., and Partridge, L. (2007). Dynamics of the action of dFOXO on adult mortality in Drosophila. Aging Cell 6, 429–438.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Golden, T. R., Hubbard, A., Dando, C., Herren, M., and Melov, S. (2008). Age-related behaviors have distinct transcriptional profiles in C. elegans. Aging Cell 7, 850–865.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Greer, E. R., Pérez, C. L., Van Gilst, M. R., Lee, B. H., and Ashrafi, K. (2008). Neural and molecular dissection of a C. elegans sensory circuit that regulates fat and feeding. Cell Metab. 8, 118–131.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Grönke, S., Clarke, D. F., Broughton, S., Andrews, T. D., and Partridge, L. (2010). Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet. 6:e1000857. doi:10.1371/journal.pgen.1000857
Ha, E., Yim, S.-V., Chung, J.-H., Yoon, K.-S., Kang, I., Cho, Y. H., et al. (2006). Melatonin stimulates glucose transport via insulin receptor substrate-1/phosphatidylinositol 3-kinase pathway in C2C12 murine skeletal muscle cells. J. Pineal Res. 41, 67–72.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Haselton, A., Sharmin, E., Schrader, J., Sah, M., Poon, P., and Fridell, Y. W. (2010). Partial ablation of adult Drosophila insulin-producing neurons modulates glucose homeostasis and extends life span without insulin resistance. Cell Cycle 9, 3063–3071.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Heidinger, B. J., Blount, J. D., Boner, W., Griffiths, K., Metcalfe, N. B., and Monaghan, P. (2012). Telomere length in early life predicts lifespan. Proc. Natl. Acad. Sci. U.S.A. 109, 1743–1748.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Hill, R. W., Wyse, G. A., and Anderson, M. (2012). Animal Physiology, 3rd Edn. Sunderland, MA: Sinauer Associates, Inc.
Ho, N., Liauw, J. A., Blaeser, F., Wei, F., Hanissian, S., Muglia, L. M., et al. (2000). Impaired synaptic plasticity and cAMP response element-binding protein activation in Ca2+/calmodulin-dependent protein kinase type IV/Gr-deficient mice. J. Neurosci. 20, 6459–6472.
Pubmed Abstract | Pubmed Full Text
Holzenberger, M., Kappeler, L., and De Magalhaes Filho, C. (2004). IGF-1 signaling and aging. Exp. Gerontol. 39, 1761–1764.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Honegger, B., Galic, M., Kohler, K., Wittwer, F., Brogiolo, W., Hafen, E., et al. (2008). Imp-L2, a putative homolog of vertebrate IGF-binding protein 7, counteracts insulin signaling in Drosophila and is essential for starvation resistance. J. Biol. 7:10. doi:10.1186/jbiol72
Hsin, H., and Kenyon, C. (1999). Signals from the reproductive system regulate the lifespan of C. elegans. Nature 399, 362–366.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Hsu, A. L., Murphy, C. T., and Kenyon, C. (2003). Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 300, 1142–1145.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Hsu, H. J., LaFever, L., and Drummond-Barbosa, D. (2008). Diet controls normal and tumorous germline stem cells via insulin-dependent and -independent mechanisms in Drosophila. Dev. Biol. 313, 700–712.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Huffman, K. (2012). The developing, aging neocortex: how genetics and epigenetics influence early developmental patterning and age-related change. Front. Genet. 3:212. doi:10.3389/fgene.2012.00212
Humphrey, D. M., Toivonen, J. M., Giannakou, M., Partridge, L., and Brand, M. D. (2009). Expression of human uncoupling protein-3 in Drosophila insulin-producing cells increases insulin-like peptide (DILP) levels and shortens lifespan. Exp. Gerontol. 44, 316–327.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Humphries, K. M., Szweda, P. A., and Szweda, L. I. (2006). Aging: a shift from redox regulation to oxidative damage. Free Radic. Res. 40, 1239–1243.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Hwangbo, D. S., Gershman, B., Tu, M. P., Palmer, M., and Tatar, M. (2004). Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature 429, 562–566.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Iser, W. B., Gami, M. S., and Wolkow, C. A. (2007). Insulin signaling in Caenorhabditis elegans regulates both endocrine-like and cell-autonomous outputs. Dev. Biol. 303, 434–447.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Jeong, D.-E., Artan, M., Seo, K., and Lee, S.-J. (2012). Regulation of lifespan by chemosensory and thermosensory systems: findings in invertebrates and their implications in mammalian aging. Front. Genet. 3:218. doi:10.3389/fgene.2012.00218
Kappeler, L., De Magalhaes Filho, C., Dupont, J., Leneuve, P., Cervera, P., Périn, L., et al. (2008). Brain IGF-1 receptors control mammalian growth and lifespan through a neuroendocrine mechanism. PLoS Biol. 6:e254. doi:10.1371/journal.pbio.0060254
Karpac, J., Hull-Thompson, J., Falleur, M., and Jasper, H. (2009). JNK signaling in insulin-producing cells is required for adaptive responses to stress in Drosophila. Aging Cell 8, 288–295.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Karpac, J., and Jasper, H. (2009). Insulin and JNK: optimizing metabolic homeostasis and lifespan. Trends Endocrinol. Metab. 20, 100–106.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Kenyon, C. (2005). The plasticity of aging: insights from long-lived mutants. Cell 120, 449–460.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Kenyon, C. (2010). The genetics of ageing. Nature 464, 504–512.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Kenyon, C., Chang, J., Gensch, E., Rudner, A., and Tabtiang, R. (1993). A C. elegans mutant that lives twice as long as wild type. Nature 366, 461–464.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Kimura, K. D., Tissenbaum, H. A., Liu, Y., and Ruvkun, G. (1997). Daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277, 942–694.
Klass, M. R. (1977). Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech. Ageing Dev. 6, 413–429.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Kourtis, N., Nikoletopoulou, V., and Tavernarakis, N. (2012). Small heat-shock proteins protect from heat-stroke-associated neurodegeneration. Nature 490, 213–218.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
la Fleur, S. E., Kalsbeek, A., Wortel, J., van der Vliet, J., and Buijs, R. M. (2001). Role for the pineal and melatonin in glucose homeostasis: pinealectomy increases night-time glucose concentrations. J. Neuroendocrinol. 13, 1025–1032.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
LaFever, L., and Drummond-Barbosa, D. (2005). Direct control of germline stem cell division and cyst growth by neural insulin in Drosophila. Science 309, 1071–1073.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Landis, J. N., and Murphy, C. T. (2010). Integration of diverse inputs in the regulation of Caenorhabditis elegans DAF-16/FOXO. Dev. Dyn. 239, 1405–1412.
Pubmed Abstract | Pubmed Full Text
Larson, K., Yan, S. J., Tsurumi, A., Liu, J., Zhou, J., Gaur, K., et al. (2012). Heterochromatin formation promotes longevity and represses ribosomal RNA synthesis. PLoS Genet. 8:e1002473. doi:10.1371/journal.pgen.1002473
Lee, J., Giordano, S., and Zhang, J. (2012). Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem. J. 441, 523–540.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Lee, K. S., Hong, S. H., Kim, A. K., Ju, S. K., Kwon, O. Y., and Yu, K. (2009). Processed short neuropeptide F peptides regulate growth through the ERK-insulin pathway in Drosophila melanogaster. FEBS Lett. 583, 2573–2577.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Lee, K. S., Kwon, O. Y., Lee, J. H., Kwon, K., Min, K. J., Jung, S. A., et al. (2008). Drosophila short neuropeptide F signalling regulates growth by ERK-mediated insulin signalling. Nat. Cell Biol. 10, 468–475.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Lee, S. J., Hwang, A. B., and Kenyon, C. (2010). Inhibition of respiration extends C. elegans life span via reactive oxygen species that increase HIF-1 activity. Curr. Biol. 20, 2131–2136.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Lee, S. J., and Kenyon, C. (2009). Regulation of the longevity response to temperature by thermosensory neurons in Caenorhabditis elegans. Curr. Biol. 19, 715–722.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Leiser, S. F., Begun, A., and Kaeberlein, M. (2011). HIF-1 modulates longevity and healthspan in a temperature-dependent manner. Aging Cell 10, 318–326.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Lemasters, J. J. (2005). Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 8, 3–5.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Leopold, P., and Perrimon, N. (2007). Drosophila and the genetics of the internal milieu. Nature 450, 186–188.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Li, W., Kennedy, S. G., and Ruvkun, G. (2003). daf-28 Encodes a C. elegans insulin superfamily member that is regulated by environmental cues and acts in the DAF-2 signaling pathway. Genes Dev. 17, 844–858.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Li, Y., and de Magalhães, J. P. (2011). Accelerated protein evolution analysis reveals genes and pathways associated with the evolution of mammalian longevity. Age (Dordr.) 35, 301–314.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Li, Y., Wang, W. J., Cao, H., Lu, J., Wu, C., Hu, F. Y., et al. (2009). Genetic association of FOXO1A and FOXO3A with longevity trait in Han Chinese populations. Hum. Mol. Genet. 18, 4897–4904.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Libert, S., Zwiener, J., Chu, X., VanVoorhies, W., Roman, G., and Pletcher, S. D. (2007). Regulation of Drosophila life span by olfaction and food-derived odors. Science 315, 1133–1137.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Libina, N., Berman, J. R., and Kenyon, C. (2003). Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell 115, 489–502.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Liesa, M., and Shirihai, O. S. (2013). Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 17, 491–506.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Liu, N., Landreh, M., Cao, K., Abe, M., Hendriks, G. J., Kennerdell, J. R., et al. (2012). The microRNA miR-34 modulates ageing and neurodegeneration in Drosophila. Nature 482, 519–523.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Loerch, P. M., Lu, T., Dakin, K. A., Vann, J. M., Isaacs, A., Geula, C., et al. (2008). Evolution of the aging brain transcriptome and synaptic regulation. PLoS ONE 3:e3329. doi:10.1371/journal.pone.0003329
Luisi, P., Alvarez-Ponce, D., Dall’Olio, G. M., Sikora, M., Bertranpetit, J., and Laayouni, H. (2012). Network-level and population genetics analysis of the insulin/TOR signal transduction pathway across human populations. Mol. Biol. Evol. 29, 1379–1392.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Maier, W., Adilov, B., Regenass, M., and Alcedo, J. (2010). A neuromedin U receptor acts with the sensory system to modulate food type-dependent effects on C. elegans lifespan. PLoS Biol. 8:e1000376. doi:10.1371/journal.pbio.1000376
Mattson, M. P. (2006). Neuronal life-and-death signaling, apoptosis, and neurodegenerative disorders. Antioxid. Redox Signal. 8, 1997–2006.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Mehta, R., Steinkraus, K. A., Sutphin, G. L., Ramos, F. J., Shamieh, L. S., Huh, A., et al. (2009). Proteasomal regulation of the hypoxic response modulates aging in C. elegans. Science 324, 1196–1198.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Morley, J. F., Brignull, H. R., Weyers, J. J., and Morimoto, R. I. (2002). The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 99, 10417–10422.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Morley, J. F., and Morimoto, R. I. (2004). Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol. Biol. Cell 15, 657–664.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Morrow, G., Samson, M., Michaud, S., and Tanguay, R. M. (2004). Overexpression of the small mitochondrial Hsp22 extends Drosophila lifespan and increases resistance to oxidative stress. FASEB J. 18, 598–609.
Pubmed Abstract | Pubmed Full Text
Murphy, C. T., Lee, S. J., and Kenyon, C. (2007). Tissue entrainment by feedback regulation of insulin gene expression in the endoderm of Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 104, 19046–19050.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Murphy, C. T., McCarroll, S., Bargmann, C., Fraser, A., Kamath, R. S., Ahringer, J., et al. (2003). Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424, 277–284.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Nabholz, B., Glémin, S., and Galtier, N. (2008). Strong variations of mitochondrial mutation rate across mammals – the longevity hypothesis. Mol. Biol. Evol. 25, 120–130.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Nakagawa, S., Lagisz, M., Hector, K. L., and Spencer, H. G. (2012). Comparative and meta-analytic insights into life-extension via dietary restriction. Aging Cell 11, 401–409.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Narendra, D., Tanaka, A., Suen, D. F., and Youle, R. J. (2008). Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 183, 795–803.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Narendra, D. P., Jin, S. M., Tanaka, A., Suen, D. F., Gautier, C. A., Shen, J., et al. (2010). PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 8:e1000298. doi:10.1371/journal.pbio.1000298
Paaby, A. B., Blacket, M. J., Hoffmann, A. A., and Schmidt, P. S. (2010). Identification of a candidate adaptive polymorphism for Drosophila life history by parallel independent clines on two continents. Mol. Ecol. 19, 760–774.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Paaby, A. B., and Schmidt, P. S. (2008). Functional significance of allelic variation at methuselah, an aging gene in Drosophila. PLoS ONE 3:e1987. doi:10.1371/journal.pone.0001987
Palikaras, K., and Tavernarakis, N. (2012). Mitophagy in neurodegeneration and aging. Front. Genet. 3:297. doi:10.3389/fgene.2012.00297
Partridge, L., and Gems, D. (2002). Mechanisms of ageing: public or private? Nat. Rev. Genet. 3, 165–175.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Partridge, L., Alic, N., Bjedov, I., and Piper, M. D. W. (2011). Ageing in Drosophila: the role of the insulin/Igf and TOR signalling network. Exp. Gerontol. 46, 376–381.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Partridge, L., Gems, D., and Withers, D. J. (2005). Sex and death: what is the connection? Cell 120, 461–472.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Pawlikowska, L., Hu, D., Huntsman, S., Sung, A., Chu, C., Chen, J., et al. (2009). Association of common genetic variation in the insulin/IGF1 signaling pathway with human longevity. Aging Cell 8, 460–472.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Pierce, S. B., Costa, M., Wisotzkey, R., Devadhar, S., Homburger, S. A., Buchman, A. R., et al. (2001). Regulation of DAF-2 receptor signaling by human insulin and ins-1, a member of the unusually large and diverse C. elegans insulin gene family. Genes Dev. 15, 672–686.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Pijpe, J., Pul, N., van Duijn, S., Brakefield, P. M., and Zwaan, B. J. (2011). Changed gene expression for candidate ageing genes in long-lived Bicyclus anynana butterflies. Exp. Gerontol. 46, 426–434.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Pincus, Z., and Slack, F. J. (2010). Developmental biomarkers of aging in Caenorhabditis elegans. Dev. Dyn. 239, 1306–1314.
Pubmed Abstract | Pubmed Full Text
Pincus, Z., Smith-Vikos, T., and Slack, F. J. (2011). MicroRNA predictors of longevity in Caenorhabditis elegans. PLoS Genet. 7:e1002306. doi:10.1371/journal.pgen.1002306
Plum, L., Schubert, M., and Bruning, J. C. (2005). The role of insulin receptor signaling in the brain. Trends Endocrinol. Metab. 16, 59–65.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Pocock, R., and Hobert, O. (2010). Hypoxia activates a latent circuit for processing gustatory information in C. elegans. Nat. Neurosci. 13, 610–614.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Poon, P. C., Kuo, T.-H., Linford, N. J., Roman, G., and Pletcher, S. D. (2010). Carbon dioxide sensing modulates lifespan and physiology in Drosophila. PLoS Biol. 8:e1000356. doi:10.1371/journal.pbio.1000356
Prahlad, V., Cornelius, T., and Morimoto, R. I. (2008). Regulation of the cellular heat shock response in Caenorhabditis elegans by thermosensory neurons. Science 320, 811–814.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Prahlad, V., and Morimoto, R. I. (2011). Neuronal circuitry regulates the response of Caenorhabditis elegans to misfolded proteins. Proc. Natl. Acad. Sci. U.S.A. 108, 14204–14209.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Puig, O., Marr, M. T. II, Ruhf, M. L., and Tjian, R. (2003). Control of cell number by Drosophila FOXO: downstream and feedback regulation of the insulin receptor pathway. Genes Dev. 17, 2006–2020.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Puig, O., and Tjian, R. (2005). Transcriptional feedback control of insulin receptor by dFOXO/FOXO1. Genes Dev. 19, 2435–2446.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Rajan, A., and Perrimon, N. (2011). Drosophila as a model for interorgan communication: lessons from studies on energy homeostasis. Dev. Cell 21, 29–31.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Rea, S., and Johnson, T. E. (2003). A metabolic model for life span determination in Caenorhabditis elegans. Dev. Cell 5, 197–203.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Rea, S. L., Ventura, N., and Johnson, T. E. (2007). Relationship between mitochondrial electron transport chain dysfunction, development, and life extension in Caenorhabditis elegans. PLoS Biol. 5:e259. doi:10.1371/journal.pbio.0050259
Reddy, P. H., and Reddy, T. P. (2011). Mitochondria as a therapeutic target for aging and neurodegenerative diseases. Curr. Alzheimer Res. 8, 393–409.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Reznick, D. N. (2005). The genetic basis of aging: an evolutionary biologist’s perspective. Sci. Aging Knowledge Environ. 2005, e7.
Rogers, C., Persson, A., Cheung, B., and de Bono, M. (2006). Behavioral motifs and neural pathways coordinating O2 responses and aggregation in C. elegans. Curr. Biol. 16, 649–659.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Rose, G., Crocco, P., De Rango, F., Montesanto, A., and Passarino, G. (2011). Further support to the uncoupling-to-survive theory: the genetic variation of human UCP genes is associated with longevity. PLoS ONE 6:e29650. doi:10.1371/journal.pone.0029650
Rose, G., Romeo, G., Dato, S., Crocco, P., Bruni, A. C., Hervonen, A., et al. (2010). Somatic point mutations in mtDNA control region are influenced by genetic background and associated with healthy aging: a GEHA study. PLoS ONE 5:e13395. doi:10.1371/journal.pone.0013395
Roth, T. L., and Sweatt, J. D. (2011). Annual research review: epigenetic mechanisms and environmental shaping of the brain during sensitive periods of development. J. Child Psychol. Psychiatry 52, 398–408.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Ruiz, M., Sanchez, D., Canal, I., Acebes, A., and Ganfornina, M. D. (2011). Sex-dependent modulation of longevity by two Drosophila homologues of human apolipoprotein D, GLaz and NLaz. Exp. Gerontol. 46, 579–589.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Rulifson, E. J., Kim, S. K., and Nusse, R. (2002). Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science 296, 1118–1120.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Rybina, O. Y., and Pasyukova, E. G. (2010). A naturally occurring polymorphism at Drosophila melanogaster Lim3 locus, a homolog of human LHX3/4, affects Lim3 transcription and fly lifespan. PLoS ONE 5:e12621. doi:10.1371/journal.pone.0012621
Saino, N., Romano, M., Ambrosini, R., Rubolini, D., Boncoraglio, G., Caprioli, M., et al. (2012). Longevity and lifetime reproductive success of barn swallow offspring are predicted by their hatching date and phenotypic quality. J. Anim. Ecol. 81, 1004–1012.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Schackwitz, W. S., Inoue, T., and Thomas, J. H. (1996). Chemosensory neurons function in parallel to mediate a pheromone response in C. elegans. Neuron 17, 719–728.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Schmidt, P. S., Duvernell, D. D., and Eanes, W. F. (2000). Adaptive evolution of a candidate gene for aging in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 97, 10861–10865.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Schroeter, H., Boyd, C. S., Ahmed, R., Spencer, J. P., Duncan, R. F., Rice-Evans, C., et al. (2003). c-Jun N-terminal kinase (JNK)-mediated modulation of brain mitochondria function: new target proteins for JNK signalling in mitochondrion-dependent apoptosis. Biochem. J. 372, 359–369.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Schubert, M., Gautam, D., Surjo, D., Ueki, K., Baudler, S., Schubert, D., et al. (2004). Role for neuronal insulin resistance in neurodegenerative diseases. Proc. Natl. Acad. Sci. U.S.A. 101, 3100–3105.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Schulz, T. J., Zarse, K., Voigt, A., Urban, N., Birringer, M., and Ristow, M. (2007). Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 6, 280–293.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Shen, J., Ford, D., Landis, G. N., and Tower, J. (2009). Identifying sexual differentiation genes that affect Drosophila life span. BMC Geriatr. 9:56 doi:10.1186/1471-2318-9-56
Shen, L. L., Du, M., Lin, X. F., Cai, T., and Wang, D. Y. (2010). Genes required for the functions of olfactory AWA neuron regulate the longevity of Caenorhabditis elegans in an insulin/IGF signaling-dependent fashion. Neurosci. Bull. 26, 91–103.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Siegal, M. L., and Bergman, A. (2002). Waddington’s canalization revisited: developmental stability and evolution. Proc. Natl. Acad. Sci. U.S.A. 99, 10528–10532.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Smith, E. D., Tsuchiya, M., Fox, L. A., Dang, N., Hu, D., Kerr, E. O., et al. (2008). Quantitative evidence for conserved longevity pathways between divergent eukaryotic species. Genome Res. 18, 564–570.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Soerensen, M., Dato, S., Christensen, K., McGue, M., Stevnsner, T., Bohr, V. A., et al. (2010). Replication of an association of variation in the FOXO3A gene with human longevity using both case-control and longitudinal data. Aging Cell 9, 1010–1017.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Stearns, S. C. (1992). The Evolution of Life Histories. Oxford: Oxford University Press.
Strand, F. L. (1999). Neuropeptides – Regulators of Physiological Processes. Cambridge, MA: MIT Press.
Suh, Y., Atzmon, G., Cho, M. O., Hwang, D., Liu, B., Leahy, D. J., et al. (2008). Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc. Natl. Acad. Sci. U.S.A. 105, 3438–3442.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Swerdlow, R. H. (2011). Brain aging, Alzheimer’s disease, and mitochondria. Biochim. Biophys. Acta 1812, 1630–1639.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Taguchi, A., Wartschow, L. M., and White, M. F. (2007). Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science 317, 369–372.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Taguchi, A., and White, M. F. (2008). Insulin-like signaling, nutrient homeostasis, and life span. Annu. Rev. Physiol. 70, 191–212.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Tatar, M. (2009). Metabolism by remote control. Cell Metab. 10, 164–166.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Tatar, M., Bartke, A., and Antebi, A. (2003). The endocrine regulation of aging by insulin-like signals. Science 299, 1346–1351.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Tazearslan, C., Ayyadevara, S., Bharill, P., and Shmookler Reis, R. J. (2009). Positive feedback between transcriptional and kinase suppression in nematodes with extraordinary longevity and stress resistance. PLoS Genet. 5:e1000452. doi:10.1371/journal.pgen.1000452
Terry, N. A., Tulina, N., Matunis, E., and DiNardo, S. (2006). Novel regulators revealed by profiling Drosophila testis stem cells within their niche. Dev. Biol. 294, 246–257.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Thyagarajan, B., Blaszczak, A. G., Chandler, K. J., Watts, J. L., Johnson, W. E., and Graves, B. J. (2010). ETS-4 is a transcriptional regulator of life span in Caenorhabditis elegans. PLoS Genet. 6:e1001125. doi:10.1371/journal.pgen.1001125
Troulinaki, K., and Bano, D. (2012). Mitochondrial deficiency: a double-edged sword for aging and neurodegeneration. Front. Genet. 3:244. doi:10.3389/fgene.2012.00244
van Ham, T. J., Holmberg, M. A., van der Goot, A. T., Teuling, E., Garcia-Arencibia, M., Kim, H. E., et al. (2010). Identification of MOAG-4/SERF as a regulator of age-related proteotoxicity. Cell 142, 601–612.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Vermehren-Schmaedick, A., Ainsley, J. A., Johnson, W. A., Davies, S.-A., and Morton, D. B. (2010). Behavioral responses to hypoxia in Drosophila larvae are mediated by atypical soluble guanylyl cyclases. Genetics 186, 183–196.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Villeponteau, B. (1997). The heterochromatin loss model of aging. Exp. Gerontol. 32, 383–394.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Volovik, Y., Maman, M., Dubnikov, T., Bejerano-Sagie, M., Joyce, D., Kapernick, E. A., et al. (2012). Temporal requirements of heat shock factor-1 for longevity assurance. Aging Cell 11, 491–499.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Walter, S., Atzmon, G., Demerath, E. W., Garcia, M. E., Kaplan, R. C., Kumari, M., et al. (2011). A genome-wide association study of aging. Neurobiol. Aging 32, 2109.e15–28.
Wang, M. C., Bohmann, D., and Jasper, H. (2005). JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell 121, 115–125.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Waskar, M., Landis, G. N., Shen, J., Curtis, C., Tozer, K., Abdueva, D., et al. (2009). Drosophila melanogaster p53 has developmental stage-specific and sex-specific effects on adult life span indicative of sexual antagonistic pleiotropy. Aging (Albany, NY) 1, 903–936.
Weindruch, R., and Walford, R. L. (1988). The Retardation of Aging and Disease by Dietary Restriction. Springfield, IL: C. C. Thomas.
Wessells, R. J., Fitzgerald, E., Cypser, J. R., Tatar, M., and Bodmer, R. (2004). Insulin regulation of heart function in aging fruit flies. Nat. Genet. 36, 1275–1281.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Willcox, B. J., Donlon, T. A., He, Q., Chen, R., Grove, J. S., Yano, K., et al. (2008). FOXO3A genotype is strongly associated with human longevity. Proc. Natl. Acad. Sci. U.S.A. 105, 13987–13992.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Williams, G. C. (1957). Pleiotropy, natural selection, and the evolution of senescence. Evolution 11, 398–411.
Wolkow, C. A., Kimura, K. D., Lee, M. S., and Ruvkun, G. (2000). Regulation of C. elegans life-span by insulinlike signaling in the nervous system. Science 290, 147–150.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Wurtman, R. J., Axelrod, J., and Fischer, J. E. (1964). Melatonin synthesis in the pineal gland: effect of light mediated by the sympathetic nervous system. Science 143, 1328–1329.
Wurtman, R. J., Axelrod, J., and Phillips, L. S. (1963). Melatonin synthesis in the pineal gland: control by light. Science 142, 1071–1073.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Wuttke, D., Connor, R., Vora, C., Craig, T., Li, Y., Wood, S., et al. (2012). Dissecting the gene network of dietary restriction to identify evolutionarily conserved pathways and new functional genes. PLoS Genet. 8:e1002834. doi:10.1371/journal.pgen.1002834
Xiao, R., Zhang, B., Dong, Y., Gong, J., Xu, T., Jianfeng, L., et al. (2013). A genetic program promotes C. elegans longevity at cold temperatures via a thermosensitive TRP channel. Cell 152, 806–817.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Yin, F., Boveris, A., and Cadenas, E. (2012). Mitochondrial energy metabolism and redox signaling in brain aging and neurodegeneration. Antioxid. Redox Signal. doi:10.1089/ars.2012.4774
Yoon, H., Enquist, L. W., and Dulac, C. (2005). Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell 123, 669–682.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Zafra, M. A., Molina, F., and Puerto, A. (2006). The neural/cephalic phase reflexes in the physiology of nutrition. Neurosci. Biobehav. Rev. 30, 1032–1044.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Zeng, Y., Cheng, L., Chen, H., Cao, H., Hauser, E. R., Liu, Y., et al. (2010). Effects of FOXO genotypes on longevity: a biodemographic analysis. J. Gerontol. A Biol. Sci. Med. Sci. 65, 1285–1299.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Zhang, C. Y., Baffy, G., Perret, P., Krauss, S., Peroni, O., Grujic, D., et al. (2001). Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell 105, 745–755.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Zhang, Y., Shao, Z., Zhai, Z., Shen, C., and Powell-Coffman, J. A. (2009). The HIF-1 hypoxia-inducible factor modulates lifespan in C. elegans. PLoS ONE 4:e6348. doi:10.1371/journal.pone.0006348
Zhou, Q., Lam, P. Y., Han, D., and Cadenas, E. (2008). c-Jun N-terminal kinase regulates mitochondrial bioenergetics by modulating pyruvate dehydrogenase activity in primary cortical neurons. J. Neurochem. 104, 325–335.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Zhou, Q., Lam, P. Y., Han, D., and Cadenas, E. (2009). Activation of c-Jun-N-terminal kinase and decline of mitochondrial pyruvate dehydrogenase activity during brain aging. FEBS Lett. 583, 1132–1140.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Keywords: aging, longevity, homeostasis, brain, nervous system, neuroendocrine system
Citation: Alcedo J, Flatt T and Pasyukova EG (2013) Neuronal inputs and outputs of aging and longevity. Front. Genet. 4:71. doi: 10.3389/fgene.2013.00071
