Proteins, nature’s versatile building blocks, offer a blueprint for designing advanced biomaterials with tailored structure, sequence, and function. This study unveils a fascinating characteristic of reflectins, a class of proteins, and their derived peptides: they exhibit distinct preferences for intracellular distribution. By dissecting the conserved motifs and flexible linkers of reflectins, we engineered a series of reflectin-derived peptides and expressed them within cells. Our findings demonstrate that the selective intracellular localization is governed by a mechanism dependent on the repetition of canonical conserved reflectin motifs (RMs). This suggests that these linkers and motifs are indeed modular fragments, ready-to-use components for synthetic design and biological construction.
To showcase the potential for precise spatiotemporal applications, we integrated RLNto2, a representative synthetic peptide derived from RfA1, into the Tet-on system. This innovative approach enabled the effective transportation of cargo peptides into cell nuclei at specific time points. Furthermore, we demonstrated that the intracellular localization of RfA1 derivatives could be spatiotemporally controlled using a CRY2/CIB1 system. Finally, we confirmed the functional homogeneity of both motifs and linkers, establishing them as standardized building blocks for synthetic biology. In essence, this research provides a modular, orthogonal, and well-characterized synthetic-peptide toolkit for precisely regulating the nucleocytoplasmic localization of proteins, paving the way for advanced applications in biotechnology and biomedicine.
1 Introduction
Peptides and their derivatives stand out as highly adaptable structural and functional units due to the vast possibilities in amino acid arrangements and combinations (Zelzer and Ulijn, 2010; Groß et al., 2016; Acar et al., 2017). Artificially designed peptides can self-assemble into diverse architectures in vitro, including fibers, tapes, tubes, sheets, and spheres (Acar et al., 2017), highlighting their considerable potential in areas such as carrier-mediated drug delivery, tissue engineering, antimicrobial agents, imaging tools, energy storage, biomineralization, and membrane protein stabilization (Mandal et al., 2014). Moreover, peptides and related derivatives have emerged as effective navigation systems for selectively targeting cellular organelles, such as the endoplasmic reticulum (Field et al., 2015; Wang et al., 2019), mitochondria (Szeto et al., 2011; Jean et al., 2016), or the nucleus (Beyer et al., 2015; Yumerefendi et al., 2015).
The precise localization of proteins is crucial for their biological functions (Itzhak et al., 2016). Therefore, transporting functional proteins or peptides to specific intracellular locations is essential for enhancing their functions in various applications (Niopek et al., 2014; Guntas et al., 2015) and for investigating their mechanisms in fundamental research (Drake et al., 2010; Slootweg et al., 2010). A prime example is the bidirectional transportation of proteins across nuclear membranes. Proteins are synthesized in the cytoplasm, yet many must be transported into the nucleus to perform their functions (Christie et al., 2016). Conversely, RNA-protein complexes need to be dynamically exported from the nucleus to the cytoplasm (Grünwald et al., 2011; Niopek et al., 2016).
Developing molecular tools to precisely regulate the movement of target proteins into and out of the nucleus is of significant value, opening up new avenues in synthetic and cell biology (Beyer et al., 2015; Niopek et al., 2016; Vogel et al., 2017).
Genetic incorporation of nuclear localization signal (NLS) sequences into cargo proteins is a common strategy for nuclear import. This approach has been successfully used to transport functional proteins (Beyer et al., 2015; Yumerefendi et al., 2015), genome-editing tools (Shi et al., 2019; Zhang et al., 2019), and transcriptional circuits (Khalil et al., 2012; Fonseca et al., 2019) into the nucleus. Similarly, nuclear export signals (NES) facilitate the translocation of molecules out of the nucleus (Beyer et al., 2015; Lerner et al., 2018). Light-responsive properties can be further introduced by integrating light-activated domains into NLS or NES, enabling reorientated trafficking (Engelke et al., 2014; Lerner et al., 2018; Allen et al., 2019). NLS sequences have also been utilized as delivery agents to enhance cellular uptake and nuclear targeting of plasmid DNA (Kim et al., 2012; Aied et al., 2013; Nematollahi et al., 2018) and other functional nanomaterials (Tammam et al., 2015; Yang et al., 2015; Yang et al., 2016). Identifying and engineering intracellular guiding sequences from natural proteins holds great promise for advancing these applications.
Reflectin proteins, uniquely found in Cephalopods (squid, octopus, and cuttlefish), are a remarkable group of functional proteins that play a key role in creating biophotonic systems and controlling structural coloration. Reflectins are highly concentrated in Bragg reflectors within iridocytes, which are responsible for iridescence through multilayer interference (Tao et al., 2010; DeMartini et al., 2013). In leucophores, reflectins are located in granular vesicles and produce bright white coloration by non-selectively reflecting all incident light (Williams et al., 2019). These intricate reflectin-based biophotonic systems have inspired the development of next-generation tunable photonic (Qin et al., 2013; Phan et al., 2015; Dennis et al., 2017) and electronic platforms and devices (Ordinario et al., 2014; Yu et al., 2014; Phan et al., 2016, although the underlying biological mechanisms remain largely unknown. Importantly, pioneering studies have explored the use of reflectins as molecular tools to modify mammalian cell functions. Recent work by Chatterjee et al. (2020) and Ogawa et al. (2020) demonstrated that expressing reflectins in human embryonic kidney (HEK) 293 cells led to the formation of phase-separated aggregates, endowing engineered human cells with tunable optical properties – a significant advancement in biosynthetic tool development and cellular function engineering (Tang et al., 2020).
Building on these insights, we utilized HEK-293T cells as a model system to investigate the biological mechanisms of reflectins and their functional potential. Complementing previous studies (Chatterjee et al., 2020; Ogawa et al., 2020), our prior research focused on understanding the extensive interactions of reflectins with cellular components (e.g., cytoskeleton) and elucidating the formation of complex biophotonic structures (Song et al., 2022). We observed that Reflectin A1, A2, B1, and C exhibit distinct cyto-/nucleoplasmic localization patterns. As natural block copolymers, reflectins are composed of positively charged polyelectrolyte linker regions (reflectin linkers, RLs) interspersed with highly conserved polyampholyte segments (reflectin motifs, RMs) (Levenson et al., 2019; Song et al., 2020). We hypothesized that specific RLs and RMs dictate the subcellular distribution of different reflectins.
Therefore, we leveraged RLs and RMs as characterized and readily available building blocks to test our hypothesis and develop a novel guiding system based on programmable RfA1 sequences. This system aims to precisely transport peptide cargoes to specific intracellular regions, either the nucleoplasm or the cytoplasm, offering a new approach to Precise Transportation of biomolecules within cells.
Our initial step involved introducing native reflectins RfA1, RfA2, RfB1, and RfC into HEK-293T cells and observing their preferential enrichment in either the nuclei or cytoplasm. Based on their sequence variations, we hypothesized that the repetition of conserved motifs is responsible for selective intracellular localization. We designed and engineered RfA1 derivatives to validate this hypothesis. We found that RLNto1, RLNto2, and RLNto3 effectively transported GFP (as a model cargo) into the nucleus, while RLNto5 led to prominent cytoplasmic enrichment of GFP. This strict intracellular localization of RfA1 derivatives confirmed the motif-repetition-dependent mechanism and suggested their potential as customizable guiding tags for transporting molecular cargoes to specific cellular regions. Subsequently, we temporally regulated the precise nuclear enrichment by integrating the Tet-On system (T Das et al., 2016; Zhou et al., 2006) with RLNto2, enabling doxycycline-controlled nuclear delivery. In this system, Tet-On components act as a “launch button,” while RfA1-derived sequences function as “guided missiles” delivering molecular cargoes to predefined targets. Finally, we synthesized genes for two recombinationally designed peptides, RMN + RM1*5 and RM1*3 + RL2*2, to verify the functional homogeneity of RMs and RLs in subcellular localization. By minimizing subtle differences among motifs or linkers in these peptides, we demonstrated that their distribution was comparable to analogous RfA1 derivatives, confirming that these peptide building blocks can be unified and standardized.
In summary, this study identified a series of modular building blocks from reflectin amino acid sequences. Recombining these blocks enabled the accurate cytoplasmic or nucleoplasmic enrichment of linked molecular cargoes (e.g., GFP), with the localization dictated by the repetition number of RMs and RLs. Combined with other synthetic biology tools, this programmable RfA1-derived strategy offers a foundation for developing spatiotemporally controllable toolkits for precise intracellular delivery, offering significant potential for biotechnology and therapeutic applications.
2 Results
2.1 Subcellular Localization Preferences of Reflectin Proteins and Deconstruction of RfA1 Sequence
Reflectin sequences are characterized by two types of patterned ∼25-amino-acid methionine-rich motifs: the N-terminal motif (RMN) [MEPMSRM(T/S)- MDF(H/Q)GR(Y/L)(I/M)DS(M/Q)(G/D)R(I/M)VDP (R/G)] and a series of conserved reflectin motifs (RMs) [M/FD(X)5MD(X)5MDX3/4] (Crookes et al., 2004) (Figure 1A and Supplementary Figure S1). The N-terminal region exhibits greater evolutionary conservation across species (Doryteuthis opalescens, Doryteuthis pealeii, Loligo forbesii, Sepia officinalis, Euprymna scolopes, and Octopus bimaculoides) and reflectin isoforms (23 in 27 known reflectins) compared to the canonical RMN (Izumi et al., 2010). Most “X” positions are predominantly occupied by a specific residue, with minor variations typically found in only one or a few reflectin motifs within the entire known reflectin library. Notably, RfC, the shortest reflectin, contains a GMXX motif and RM*. The GMXX motif is a unique hydrophobic region with a four-amino-acid repeat where ‘X’ represents less conserved locations. The asterisk-marked RM* in RfC shows significant sequence deviations not seen in other reflectin motifs (Levenson et al., 2017). Supplementary Figure S1 illustrates reflectins and their RMN and RMs.
FIGURE 1
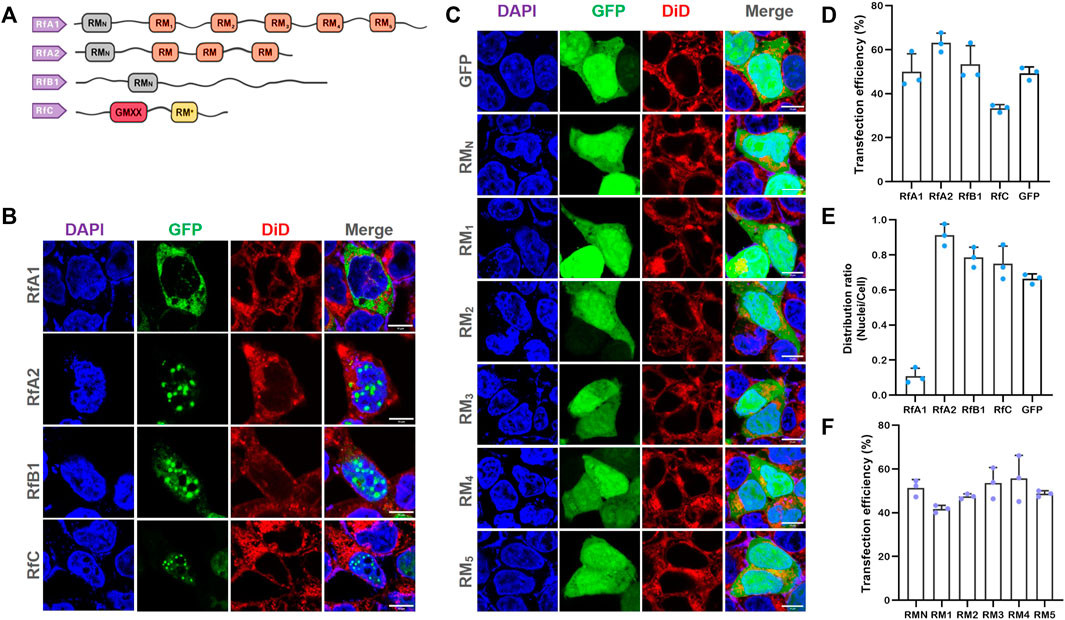 Selective Intracellular Localization of Reflectins and Free-Distribution of Single Motifs
Selective Intracellular Localization of Reflectins and Free-Distribution of Single Motifs
FIGURE 1. Selective Intracellular Localization of Reflectins and Free-Distribution of Single Motifs. (A) Schematics of reflectin protein sequences. Conserved Reflectin Motifs (RMN and repeated RM1-5) are shown as boxes, and reflectin linkers (RLs) as lines. (B,C) Fluorescence microscopy images of transfected HEK-293T cells stained with DAPI and DiD. Reflectins and variants are visualized using tandem EGFP. Scale bar = 10 μm. (D) Cell number statistics and quantification of transfection efficiencies for four reflectins and GFP. (E) Distribution ratio statistics of fluorescent intensity in transfected cells and their nuclei. (F) Cell number statistics and quantification of transfection efficiencies for five single RMs. Data are presented as mean values ±SD for n = 3 independent experiments.
Current research primarily focuses on the in vitro self-assembly properties of reflectins (DeMartini et al., 2015; Levenson et al., 2016; Levenson et al., 2019). These dynamic assembly properties have inspired the development of advanced tunable photonic (Qin et al., 2013; Phan et al., 2015; Dennis et al., 2017) and electronic platforms and devices (Ordinario et al., 2014; Yu et al., 2014; Phan et al., 2016).
To further investigate their intracellular behavior, we introduced four reflectin proteins into HEK 293T cells using pEGFP-C1 vectors. Compared to cells transfected with empty pEGFP-C1, cells expressing reflectins showed a tendency to form protein condensates or spherical droplets (Supplementary Figure S2 for large-area immunofluorescence images and Supplementary Figure S3 for cell viability data from CCK-8 assays). The formation of these proteinaceous condensates supports the hypothesis that reflectins are intrinsically disordered proteins capable of phase separation (Levenson et al., 2019).
Notably, RfA1 condensates were predominantly localized in the cytoplasm, while RfA2, RfB1, and RfC droplets were highly enriched in the nuclei (Figures 1B, E). Amino acid composition dictates protein structure and function, and the similarities and differences among reflectins are determined by their primary structures. Reflectins are grouped into one protein family due to shared reflectin motifs (RMs). However, the most significant variation among reflectins is the number of RM repetitions. As suggested by Morse and colleagues, these canonical reflectin motifs (RMs) may function as structural or functional elements (Levenson et al., 2016). Cytoplasm-localized RfA1 contains the highest number of reflectin motifs, while nucleoplasm-enriched reflectins have fewer. To investigate the role of conserved RMs in protein condensation and selective localization, we cloned the six RMs of RfA1 (RMN, RM1, 2, 3, 4, 5; primers in Supplementary Table S1) and expressed them individually in cells. The results revealed that all single RMs distributed freely throughout both the cytoplasm and nucleoplasm, showing no difference from cells expressing GFP alone (Figure 1C). This indicates that the cytoplasmic enrichment of reflectins, beyond conserved amino acid composition, is likely driven by their segmented sequence structure. Transfection efficiencies are shown in Figures 1D and 1F.
2.2 Reconstruction of Block Amino Acid Sequence and Intracellular Localization
We designed five pairs of primers to clone DNA sequences from RfA1 genes, progressively extending peptide sequences and reconstructing their segmented structure (primers in Supplementary Table S2). The PCR products encoding RLNto1, RLNto2, RLNto3, RLNto4, and RLNto5 were subsequently cloned into the pEGFP-C1 vector. Cells expressing RLNto1, RLNto2, and RLNto3 shared a common characteristic: enrichment in the nuclei (Figures 2A, E). This localization pattern differed from the cytoplasmic preference of RfA1 and the free distribution of single RMs, but resembled the localization of simpler reflectins (RfA2, RfB1, and RfC). Furthermore, RLNto2 and RLNto3 were also enriched in the cytoplasm but excluded from the most crowded cellular regions (Figures 2A, E). In contrast, longer peptides, RLNto4 and RLNto5, began to be excluded from the nuclei and formed condensates in the cytoplasm (Figures 2A, E), closely mirroring the localization of RfA1 (Figures 1B, E).
FIGURE 2
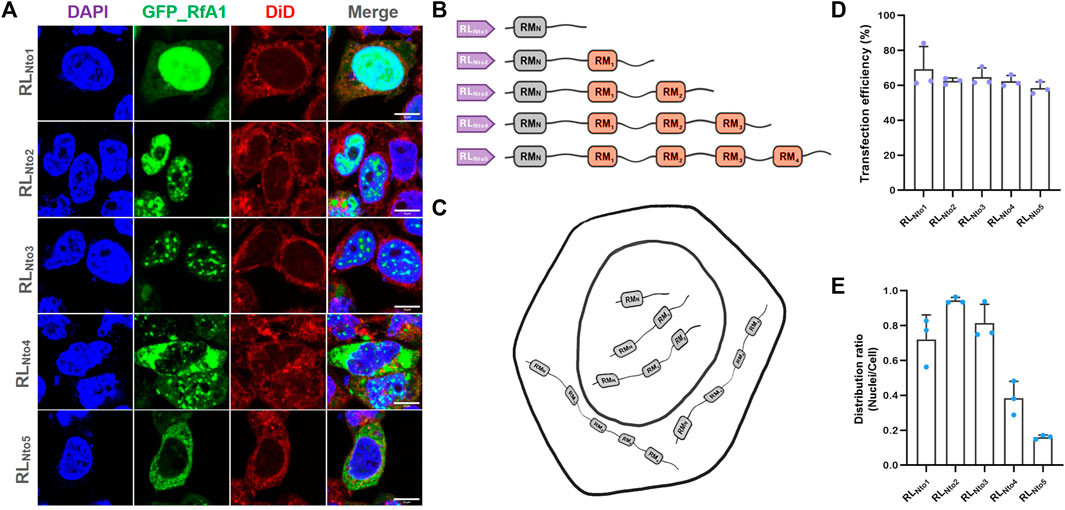 Recurrence of phase separation and cyto-/nucleo-localization preferences of RfA1-derived peptides in fixed HEK-293T cells
Recurrence of phase separation and cyto-/nucleo-localization preferences of RfA1-derived peptides in fixed HEK-293T cells
FIGURE 2. Recurrence of phase separation and cyto-/nucleo-localization preferences of RfA1-derived peptides in fixed HEK-293T cells. (A) Nuclei and membranes are stained with DAPI (blue) and DiD (red), respectively. RfA1-derived peptides are visualized with tandem EGFP (green). Scale bars = 10 μm. (B) Schematics of RfA1 derivatives. (C) Illustration of subcellular localization of RLNto1, RLNto2, RLNto3, RLNto4, and RLNto5. (D) Cell number statistics and quantification of transfection efficiencies of RLNtox (x = 1, 2, 3, 4, and 5). (E) Distribution ratio statistics of fluorescent intensity in transfected cells and their nuclei (Supplementary Figure S4 for large-area immunofluorescence images and Supplementary Figure S3 for cell viability data from CCK-8 assays). Data are presented as mean values ±SD for n = 3 independent experiments.
Guan et al. (2017) proposed that reflectin motifs may originate from a 24-bp transposon-like DNA fragment from the symbiotic bioluminescent bacterium Vibrio fischeri. They suggested that millions of years of self-replication and translocation of this transposon led to the formation of reflectin motifs and the diverse reflectin family. Our findings support Guan’s evolutionary hypothesis, as the repetition number of RMs, the fundamental units, precisely determines the distinct properties, including intracellular localization, among RfA1, A2, B1, and C. The 24-bp transposon-like DNA fragment, as a subordinate element and evolutionary origin, is likely the root of reflectin diversification.
Furthermore, if GFP is considered a molecular cargo, reflectin derivatives can be viewed as intelligent vehicles for delivering cargoes to pre-selected destinations (cytoplasm or nucleoplasm). Based on this concept, we explored the potential of RfA1 variants as synthetic biology tools. RLNto2, the shortest peptide that strictly targets nuclei, was selected for subsequent studies as a guiding tag. We optimized transfection conditions through dose-dependent and time-scale preliminary assays (Supplementary Figure S5).
2.3 Doxycycline-Induced Tet-On System for Temporal Control of RLNto2
To demonstrate the potential of RLNto2 as a synthetic biology component with temporal control, we integrated it into the Tet-On system, which can be activated or deactivated by doxycycline (dox) (Figure 3A). Transfection was performed using Lipofectamine 3000 protocol when cell confluence reached approximately 30%. Transfection efficiency was assessed by fluorescence microscopy after 24 hours. Cells were then treated with varying concentrations of dox and cultured for an additional 24 hours. Confocal microscopy revealed the nuclei-targeted expression of RLNto2 upon dox induction (Figures 3B, C and Supplementary Figure S6). The expression level of RLNto2 increased proportionally with dox concentration (Figure 3C), indicating successful activation and dose-dependent controllability of the Tet-On system.
FIGURE 3
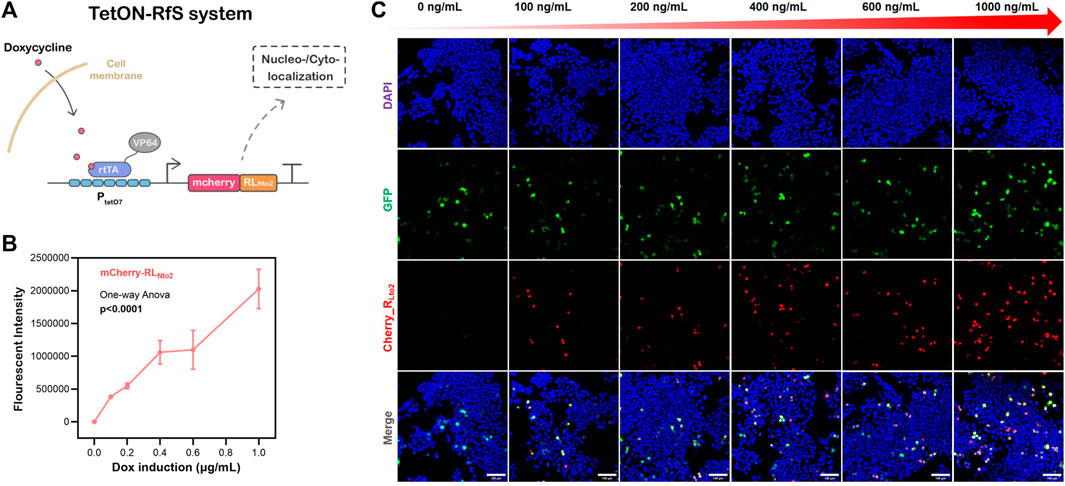 Dose-dependent nuclei-targeted Tet-On-RLNto2 system mediated by doxycycline
Dose-dependent nuclei-targeted Tet-On-RLNto2 system mediated by doxycycline
FIGURE 3. Dose-dependent nuclei-targeted Tet-On-RLNto2 system mediated by doxycycline. (A) Schematic diagram of the Tet-On-Rfs system. (B) Total Intensity, representing the overall expression of mCherry_RLNto2. Data are presented as mean values ±SD for n = 3 independent experiments. (C) Activation of the Tet-On-Rfs system. Transfected cells are indicated by GFP (green), and the localization of RLNto2 is labeled by tandem mCherry (Red). Scale bars = 100 μm.
It is foreseeable that replacing the mCherry reporter gene with other functional or therapeutic peptides would allow the Tet-On-Rfs system to precisely deliver molecular cargoes into nuclei, amplifying their biological effects. Furthermore, for applications requiring cytoplasmic localization, RLNto2 could be substituted with RLNto4 or RLNto5. This programmable RfA1 sequence-based toolkit provides customizable and selectable options for precise transportation of proteins or peptide cargoes to specific subcellular regions.
2.4 Blue-Light-Controlled System for Spatiotemporal Subcellular Enrichment
Based on our observations in Figure 2, RfA1-derived peptides with more than 3 RM motifs tend to reside in the cytoplasm, while shorter peptides are inclined to accumulate in the nucleoplasm. We hypothesized that while RLNto2 can traverse nuclear membranes, its dimer or analog might be restricted. To test this, we introduced CoH2/DocS domains, known for their stable and high-affinity interaction (Li et al., 2020; Yu et al., 2020).
Recombinant proteins mCherry-RLNto2-CoH2 and GFP-RLNto2 could both enter the nuclei (Figure 4A). Sharing the same transmembrane mechanism, these proteins exhibited similar nuclear responses and co-localized. Co-expression of mCherry-RLNto2 and GFP-RLNto2-DocS resulted in the same phenomenon (Figure 4B). However, when CoH2 and DocS domains were fused to mCherry-RLNto2 and GFP-RLNto2, respectively, the majority of the fluorescence signal was retained in the cytoplasm (Figure 4C). These results suggest that the repetition-dependent nucleocytoplasmic localization preference of RfA1-derived peptides can be modulated through molecular splicing. Inspired by this, we employed the CRY2–CIB1 system to create a photoactivatable subcellular localization system. In the absence of blue-light stimulation, mCherry-RLNto2-CIB1 and GFP-RLNto2-CRY2 tended to enrich in nuclei and co-localize (Figure 4D). Conversely, blue-light irradiation prevented the green fluorescent signal of GFP-RLNto2-CRY2 from entering the nuclei in a subset of cells (Figure 4E). In this scenario, blue-light-induced interaction between CIB1 and CRY2 effectively increased the RM repetition level, as RLNto2 effectively formed a dimer analog RLNto2-Pairs-RLNto2, leading to cytoplasmic retention (Figure 4F).
FIGURE 4
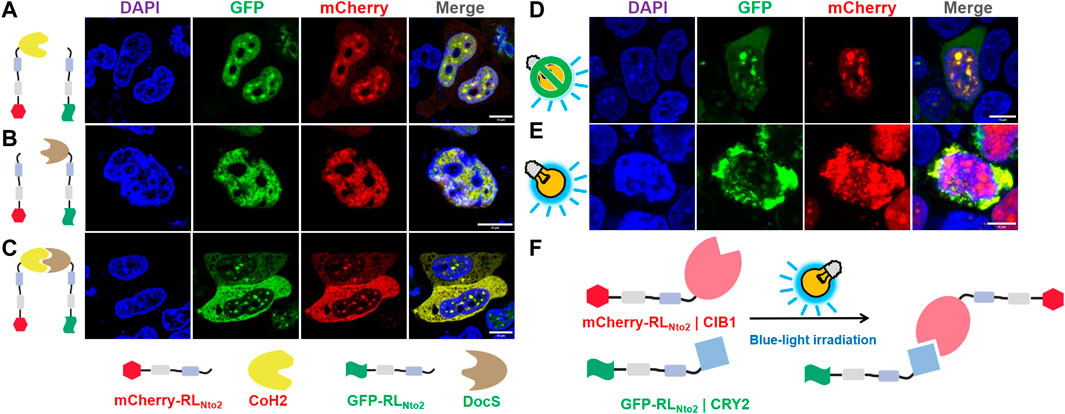 Restoration of repetition-level dependent cytoplasmic enrichment
Restoration of repetition-level dependent cytoplasmic enrichment
FIGURE 4. Restoration of repetition-level dependent cytoplasmic enrichment. (A) Confocal microscopy of HEK-29ET cells transfected with pCDNA3.1-mCherry-RLNto2-CoH2 and pCDNA3.1-GFP-RLNto2. (B) Confocal microscopy of HEK-29ET cells transfected with pCDNA3.1-mCherry-RLNto2 and pCDNA3.1-GFP-RLNto2-DocS. (C) Confocal microscopy of HEK-29ET cells transfected with pCDNA3.1-mCherry-RLNto2-CoH2 and pCDNA3.1-GFP-RLNto2-DocS. (D,E) Confocal microscopy of HEK-29ET cells transfected with CDNA3.1-mCherry-RLNto2-CIB1 and pCDNA3.1-GFP-RLNto2-CRY2, treated with or without blue light, respectively. (F) Illustration of the blue-light-induced interaction between recombinant mCherry-RLNto2-CIB1 and GFP-RLNto2-CRY2. Scale bars = 10 μm.
2.5 Standardization of Reflectin-Derived Building Blocks
For synthetic biology applications, building blocks should ideally be standardized and modular. Although reflectin RMs are highly conserved, subtle compositional variations exist among them (Crookes et al., 2004; Izumi et al., 2010; Levenson et al., 2017). To eliminate this compositional discrepancy, we replaced all RM1∼5 in RfA1 with a uniform RM1 (sequence information in Supplementary Table S3 and schematics in Figure 5B). Similar to native RfA1, RMN + RM1*5 also exhibited strong cytoplasmic enrichment (Figures 5A, E). This unification of RM1∼5 into RM1 did not alter the protein’s intracellular localization preference. Furthermore, we designed a recombinational peptide RM1*3 + RL2*2 (sequence information in Supplementary Table S3; schematics in Figure 5D). This peptide was transported to and enriched in nuclei (Figure 5C), mirroring the localization of the RfA1-derived analog RLNto2 (Supplementary Figure S6C, E). These results demonstrate that functional components derived from reflectin amino acid sequences can be standardized without losing their intracellular localization properties, making them suitable for synthetic biology applications.
FIGURE 5
 Homogeneity/standardization of reflectin-derived building blocks
Homogeneity/standardization of reflectin-derived building blocks
FIGURE 5. Homogeneity/standardization of reflectin-derived building blocks. (A,C) Fluorescence images of fixed HEK-293T cells transfected with pEGFP-C1-(RMN + RM1*5) and pEGFP-C1-(RM1*3 + RL2*2), respectively. Nuclei and cytomembranes are stained with DAPI and DiD. (B,D) Schematics of RMN + RM1*5 and RM1*3 + RL2*2 sequences. (E) Distribution ratio statistics of fluorescent intensity in transfected cells and their nuclei. Data are presented as mean values ±SD for n = 3 independent experiments.
3 Discussion
The precise localization of proteins is essential for proper organelle function (Itzhak et al., 2016). While proteins are translated in the cytoplasm, many need to be transported to the nucleus to carry out their functions (Christie et al., 2016). Conversely, the dynamic export of RNA-protein complexes from the nucleus is a crucial, yet incompletely understood, process in molecular biology (Grünwald et al., 2011; Niopek et al., 2016). Therefore, molecular tools that can qualitatively and quantitatively regulate the entry and exit of target proteins into and out of the nucleus are invaluable for advancing synthetic and cell biology (Beyer et al., 2015; Niopek et al., 2016; Vogel et al., 2017).
This study revealed distinct intracellular localization preferences among different reflectin proteins. Based on this characteristic, we designed a series of synthetic peptides capable of precise targeting to preselected subcellular locations. Furthermore, by integrating reflectin-derived peptides with established cell biology tools, we demonstrated that the selectable enrichment of these peptides and their conjugated molecular cargoes can be temporally and spatially controlled through chemical or light stimuli, highlighting their potential as biosynthetic elements for precise transportation.
Intrigued by their intracellular functions and properties, we introduced genes for four native reflectins (RfA1, RfA2, RfB1, and RfC) into HKE-293T cells. We observed distinct localization preferences: RfA1 was predominantly cytoplasmic, whereas RfA2, RfB1, and RfC were highly enriched in the nucleoplasm. As natural block copolymers, reflectins consist of positively charged polyelectrolyte linker regions (RLs) interspersed with highly conserved polyampholyte segments (RMs). The key difference among reflectins is the number and arrangement of RMs.
Therefore, reflectin sequences offer programmable building blocks for guiding cargo molecules and achieving selective subcellular localization for biosynthetic applications. We used RfA1 as a template, systematically removing RMs from its amino acid sequence via gene engineering. We found that longer RfA1 derivatives with more RM repeats tended to remain in the cytoplasm, while shorter truncations began to enter the nucleus. We further integrated RfA1-derived guiding peptides with the Tet-On system. In this demonstration, Tet-On elements served as a trigger, enabling precise activation at a scheduled time point under doxycycline regulation. Simultaneously, RfA1 derivative RLNto2 acted as a precision-guided system, effectively delivering molecular cargoes (e.g., mCherry) into the nucleus, showcasing precise transportation capabilities.
The repetition-dependent nucleocytoplasmic localization preference of RfA1-derived peptides could be modulated through molecular splicing. Increasing the RM repetition level, by creating a dimer-analog RLNto2-CoH2/DocS-RLNto2, prevented nuclear entry and retained these combined proteins in the cytoplasm. Similarly, by replacing the CoH2/DocS system with a blue-light-regulated CRY2/CIB1 system, we achieved controllable subcellular localization of RfA1-derived peptides using light. These results strongly indicate the versatility and expandability of the RfA1-derived molecular toolkit, allowing integration with other synthetic biology systems for precise transportation. However, further optimization is needed to precisely regulate this blue-light-controlled intracellular distribution system, including optimizing plasmids ratios, light intensity, and treatment duration. Given that the blue-light receptor cryptochrome undergoes oligomerization upon blue-light signal transduction (Ma et al., 2020), the blue-light-induced cytoplasmic retention of RLNto2-CRY2/CIB1-RLNto2 may involve more complex mechanisms that require further investigation for full understanding.
Finally, we validated the functional homogeneity of RMs and RLs by replacing RM2, 3, 4, 5 in RfA1 with a uniform RM1 and by recombinationally designing an artificial peptide “RM1*3 + RL2*2.” Guiding sequences derived from the RfA1 amino acid sequence can be modified into unified and standardized building blocks for cyto- or nucleo-targeting. The RfA1-derived strategy and standardized building blocks can be further programmed and developed into versatile and spatiotemporally controllable toolkits when combined with other responsive synthetic biology components, offering a powerful approach to precise transportation within cellular environments.
4 Methods
4.1 Construction of Recombinant pEGFP-C1 Vectors
The nucleotide sequences of D. (Loligo) pealeii reflectin A1 (RfA1) (Genbank: ACZ57764.1), D. (Loligo) pealeii reflectin A2 (RfA2) (Genbank: ACZ57765.1), D. (Loligo) pealeii reflectin B1 (RfB1) (Genbank: ACZ57766), and D. Opalescens reflectin C (Genbank: AIN36559.1) were codon-optimized for human-cell expression. Sangon Biotech® (Shanghai, China) synthesized and sequence-verified these optimized genes. Primers (F-GAATTCTATGAATAGATATTTGAATAGACA; R-GGATCCATACATATGATAATCATAATA ATTT) were designed to introduce EcoR I and BamH I restriction sites, enabling the cloning of the modified RfA1 CDS into pEGFP-C1 via standard restriction enzyme cloning. For truncated RfA1 derivatives, six pairs of primers were used. For instance, selecting RMN-F and RM3-R primers resulted in a nucleotide sequence encoding RMN-RL1-RM1-RL2-RM2-RL3-RM3 (referred to as RMNto3 in this study) after PCR. During primer synthesis, 5′GCATGGACGAGCTGTACAAG 3′ and 5′ TTATGATCAG- TTATCTAGAT 3’ sequences were added to the F-primers and R-primers, respectively, to facilitate ligation into pEGFP-C1 using the Ready-to-Use Seamless Cloning Kit from Sangon Biotech® (Shanghai, China).
4.2 Growth and Transfection of Human Cells
HEK-293T cells (ATCC®, CRL-3216TM) were cultured in Dulbecco’s Modified Eagle Medium (DMEM, GibcoTM) supplemented with 10% fetal bovine serum (FBS, GibcoTM) at 37°C under 5% CO2. For glass bottom dishes from Cellvis (California, United States), cells were seeded at approximately 33% confluency one day before transfection and cultured for another 24 hours. Transfection mixtures containing Lipofectamine 3000 (Thermo Scientific) and recombinant vectors were added to the medium and incubated for ~16–24 hours. For CCK-8 assays, 1 × 10^4 cells were seeded into each well of 96-well plates one day before transfection. Cells were transfected with recombinant vectors and incubated for another 24 hours. Subsequently, 10 μL of CCK-8 solution was added to each well for a ~2–4 hour chromogenic reaction. OD450 was measured using a Multiskan FC (Thermo Scientific).
4.3 Fluorescence Microscopy of Stained Cells
Transfected HEK-293T cells grown in Cellvis plastic dishes were initially fixed with 4% paraformaldehyde at room temperature for 30 minutes and then stained with DiD (diluted in 0.5% Triton X-100 PBS) for ~30 minutes after PBS washes. After washing off the fluorescent dye with PBS, fixed cells were mounted in DAPI-Fluoromount (Beyotime, Shanghai, China) and imaged using a Leica TCS SP8 imaging system in fluorescence mode. DAPI detection used a 405 Diode laser, GFP detection used a 488 Argon laser, mCherry detection used a DPSS 561 laser, and DiD detection used a HeNeB 633 laser. Image analysis was performed using ImageJ (Java 1.8.0_172/1.52b) (Schindelin et al., 2012).
Data availability statement
The datasets generated and analyzed in this study are available in online repositories. Repository information and accession number(s) are provided within the article and Supplementary Material.
Author contributions
JS and CL: Conceptualization, methodology, experimentation, data analysis, manuscript writing. LZe: Graduate student guidance, experiment assistance. BL and LL: Experimentation. ZY: Data analysis. WW, LZh, and BH: Experiment design, manuscript writing, funding acquisition.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 31971291 and 32201136) and the Natural Science Foundation of Hunan Province (Grant No. 2020JJ5655).
Acknowledgments
JS expresses gratitude to Prof. D. E. Morse and R. Levenson (MCDB, UCSB) for their mentorship from 2016 to 2018.
Conflict of interest
The authors declare no competing financial interests.
Publisher’s note
All claims presented in this article are solely those of the authors and do not necessarily represent the views of their affiliated organizations, or those of the publisher, editors, and reviewers. Any product evaluated or claim made by its manufacturer is not guaranteed or endorsed by the publisher.
Supplementary material
Supplementary Material for this article is available online at: https://www.frontiersin.org/articles/10.3389/fbioe.2023.1062769/full#supplementary-material
References
[#B1] Acar, H., Srivastava, S., Chung, E. J., Schnorenberg, M. R., Barrett, J. C., LaBelle, J. L., et al. (2017). Self-assembling peptide-based building blocks in medical applications. *Adv. Drug Deliv. Rev.* 110-111, 65–79. doi:10.1016/j.addr.2016.08.006
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/27535485/) | [CrossRef Full Text](https://doi.org/10.1016/j.addr.2016.08.006) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Self-assembling+peptide-based+building+blocks+in+medical+applications&btnG=)
[#B2] Aied, A., Greiser, U., Pandit, A., and Wang, W. (2013). Polymer gene delivery: Overcoming the obstacles. *Drug Discov. Today* 18, 1090–1098. doi:10.1016/j.drudis.2013.06.014
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/23831858/) | [CrossRef Full Text](https://doi.org/10.1016/j.drudis.2013.06.014) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Polymer+gene+delivery:+Overcoming+the+obstacles&btnG=)
[#B3] Allen, M. E., Zhou, W., Thangaraj, J., Kyriakakis, P., Wu, Y., Huang, Z., et al. (2019). An AND-gated drug and photoactivatable cre-loxP system for spatiotemporal control in cell-based therapeutics. *ACS Synth. Biol.* 8, 2359–2371. doi:10.1021/acssynbio.9b00175
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/31592660/) | [CrossRef Full Text](https://doi.org/10.1021/acssynbio.9b00175) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=An+AND-gated+drug+and+photoactivatable+cre-loxP+system+for+spatiotemporal+control+in+cell-based+therapeutics&btnG=)
[#B4] Beyer, H. M., Juillot, S., Herbst, K., Samodelov, S. L., Muller, K., Schamel, W. W., et al. (2015). Red light-regulated reversible nuclear localization of proteins in mammalian cells and zebrafish. *ACS Synth. Biol.* 4, 951–958. doi:10.1021/acssynbio.5b00004
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/25803699/) | [CrossRef Full Text](https://doi.org/10.1021/acssynbio.5b00004) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Red+light-regulated+reversible+nuclear+localization+of+proteins+in+mammalian+cells+and+zebrafish&btnG=)
[#B5] Chatterjee, A., Cerna Sanchez, J. A., Yamauchi, T., Taupin, V., Couvrette, J., and Gorodetsky, A. A. (2020). Cephalopod-inspired optical engineering of human cells. *Nat. Commun.* 11, 2708. doi:10.1038/s41467-020-16151-6
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/32488070/) | [CrossRef Full Text](https://doi.org/10.1038/s41467-020-16151-6) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Cephalopod-inspired+optical+engineering+of+human+cells&btnG=)
[#B6] Christie, M., Chang, C.-W., Róna, G., Smith, K. M., Stewart, A. G., Takeda, A. A., et al. (2016). Structural biology and regulation of protein import into the nucleus. *J. Mol. Biol.* 428, 2060–2090. doi:10.1016/j.jmb.2015.10.023
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/26523678/) | [CrossRef Full Text](https://doi.org/10.1016/j.jmb.2015.10.023) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Structural+biology+and+regulation+of+protein+import+into+the+nucleus&btnG=)
[#B7] Crookes, W. J., Ding, L.-L., Huang, Q. L., Kimbell, J. R., Horwitz, J., and McFall-Ngai, M. J. (2004). Reflectins: The unusual proteins of squid reflective tissues. *Science* 303, 235–238. doi:10.1126/science.1091288
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/14716016/) | [CrossRef Full Text](https://doi.org/10.1126/science.1091288) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Reflectins:+The+unusual+proteins+of+squid+reflective+tissues&btnG=)
[#B8] DeMartini, D. G., Izumi, M., Weaver, A. T., Pandolfi, E., and Morse, D. E. (2015). Structures, organization, and function of reflectin proteins in dynamically tunable reflective cells. *J. Biol. Chem.* 290, 15238–15249. doi:10.1074/jbc.m115.638254
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/25918159/) | [CrossRef Full Text](https://doi.org/10.1074/jbc.m115.638254) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Structures,+organization,+and+function+of+reflectin+proteins+in+dynamically+tunable+reflective+cells&btnG=)
[#B9] DeMartini, D. G., Krogstad, D. V., and Morse, D. E. (2013). Membrane invaginations facilitate reversible water flux driving tunable iridescence in a dynamic biophotonic system. *Proc. Natl. Acad. Sci. U. S. A.* 110, 2552–2556. doi:10.1073/pnas.1217260110
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/23359694/) | [CrossRef Full Text](https://doi.org/10.1073/pnas.1217260110) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Membrane+invaginations+facilitate+reversible+water+flux+driving+tunable+iridescence+in+a+dynamic+biophotonic+system&btnG=)
[#B10] Dennis, P. B., Singh, K. M., Vasudev, M. C., Naik, R. R., and Crookes-Goodson, W. J. (2017). Research update: A minimal region of squid reflectin for vapor-induced light scattering. *Apl. MATER* 5, 120701. doi:10.1063/1.4997199
[CrossRef Full Text](https://doi.org/10.1063/1.4997199) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Research+update:+A+minimal+region+of+squid+reflectin+for+vapor-induced+light+scattering&btnG=)
[#B11] Drake, K. R., Kang, M., and Kenworthy, A. K. (2010). Nucleocytoplasmic distribution and dynamics of the autophagosome marker EGFP-LC3. *PloS one* 5, e9806. doi:10.1371/journal.pone.0009806
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/20352102/) | [CrossRef Full Text](https://doi.org/10.1371/journal.pone.0009806) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Nucleocytoplasmic+distribution+and+dynamics+of+the+autophagosome+marker+EGFP-LC3&btnG=)
[#B12] Engelke, H., Chou, C., Uprety, R., Jess, P., and Deiters, A. (2014). Control of protein function through optochemical translocation. *ACS Synth. Biol.* 3, 731–736. doi:10.1021/sb400192a
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/24933258/) | [CrossRef Full Text](https://doi.org/10.1021/sb400192a) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Control+of+protein+function+through+optochemical+translocation&btnG=)
[#B13] Field, L. D., Delehanty, J. B., Chen, Y., and Medintz, I. L. (2015). Peptides for specifically targeting nanoparticles to cellular organelles: Quo vadis? *Accounts Chem. Res.* 48, 1380–1390. doi:10.1021/ar500449v
[CrossRef Full Text](https://doi.org/10.1021/ar500449v) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Peptides+for+specifically+targeting+nanoparticles+to+cellular+organelles:+Quo+vadis?&btnG=)
[#B14] Fonseca, J. P., Bonny, A. R., Kumar, G. R., Ng, A. H., Town, J., Wu, Q. C., et al. (2019). A toolkit for rapid modular construction of biological circuits in mammalian cells. *ACS Synth. Biol.* 8, 2593–2606. doi:10.1021/acssynbio.9b00322
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/31686495/) | [CrossRef Full Text](https://doi.org/10.1021/acssynbio.9b00322) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=A+toolkit+for+rapid+modular+construction+of+biological+circuits+in+mammalian+cells&btnG=)
[#B15] Groß, A., Hashimoto, C., Sticht, H., and Eichler, J. (2016). Synthetic peptides as protein mimics. *Front. Bioeng. Biotechnol.* 3, 211. doi:10.3389/fbioe.2015.00211
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/26835447/) | [CrossRef Full Text](https://doi.org/10.3389/fbioe.2015.00211) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Synthetic+peptides+as+protein+mimics&btnG=)
[#B16] Grünwald, D., Singer, R. H., and Rout, M. (2011). Nuclear export dynamics of RNA–protein complexes. *Nature* 475, 333–341. doi:10.1038/nature10318
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/21776079/) | [CrossRef Full Text](https://doi.org/10.1038/nature10318) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Nuclear+export+dynamics+of+RNA%E2%80%93protein+complexes&btnG=)
[#B17] Guan, Z., Cai, T., Liu, Z., Dou, Y., Hu, X., Zhang, P., et al. (2017). Origin of the reflectin gene and hierarchical assembly of its protein. *Curr. Biol.* 27, 2833–2842.e6. doi:10.1016/j.cub.2017.07.061
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/28889973/) | [CrossRef Full Text](https://doi.org/10.1016/j.cub.2017.07.061) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Origin+of+the+reflectin+gene+and+hierarchical+assembly+of+its+protein&btnG=)
[#B18] Guntas, G., Hallett, R. A., Zimmerman, S. P., Williams, T., Yumerefendi, H., Bear, J. E., et al. (2015). Engineering an improved light-induced dimer (iLID) for controlling the localization and activity of signaling proteins. *Proc. Natl. Acad. Sci.* 112, 112–117. doi:10.1073/pnas.1417910112
[CrossRef Full Text](https://doi.org/10.1073/pnas.1417910112) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Engineering+an+improved+light-induced+dimer+(iLID)+for+controlling+the+localization+and+activity+of+signaling+proteins&btnG=)
[#B19] Itzhak, D. N., Tyanova, S., Cox, J., and Borner, G. H. (2016). Global, quantitative and dynamic mapping of protein subcellular localization. *Elife* 5, e16950. doi:10.7554/eLife.16950
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/27278775/) | [CrossRef Full Text](https://doi.org/10.7554/eLife.16950) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Global,+quantitative+and+dynamic+mapping+of+protein+subcellular+localization&btnG=)
[#B20] Izumi, M., Sweeney, A. M., DeMartini, D., Weaver, J. C., Powers, M. L., Tao, A., et al. (2010). Changes in reflectin protein phosphorylation are associated with dynamic iridescence in squid. *J. R. Soc. Interface* 7, 549–560. doi:10.1098/rsif.2009.0299
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/19776150/) | [CrossRef Full Text](https://doi.org/10.1098/rsif.2009.0299) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Changes+in+reflectin+protein+phosphorylation+are+associated+with+dynamic+iridescence+in+squid&btnG=)
[#B21] Jean, S. R., Ahmed, M., Lei, E. K., Wisnovsky, S. P., and Kelley, S. O. (2016). Peptide-mediated delivery of chemical probes and therapeutics to mitochondria. *Accounts Chem. Res.* 49, 1893–1902. doi:10.1021/acs.accounts.6b00277
[CrossRef Full Text](https://doi.org/10.1021/acs.accounts.6b00277) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Peptide-mediated+delivery+of+chemical+probes+and+therapeutics+to+mitochondria&btnG=)
[#B22] Khalil, A. S., Lu, T. K., Bashor, C. J., Ramirez, C. L., Pyenson, N. C., Joung, J. K., et al. (2012). A synthetic biology framework for programming eukaryotic transcription functions. *Cell* 150, 647–658. doi:10.1016/j.cell.2012.05.045
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/22863014/) | [CrossRef Full Text](https://doi.org/10.1016/j.cell.2012.05.045) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=A+synthetic+biology+framework+for+programming+eukaryotic+transcription+functions&btnG=)
[#B23] Kim, B. K., Kang, H., Doh, K. O., Lee, S. H., Park, J. W., Lee, S. J., et al. (2012). Homodimeric SV40 NLS peptide formed by disulfide bond as enhancer for gene delivery. *Bioorg Med. Chem. Lett.* 22, 5415–5418. doi:10.1016/j.bmcl.2012.07.051
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/22871581/) | [CrossRef Full Text](https://doi.org/10.1016/j.bmcl.2012.07.051) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Homodimeric+SV40+NLS+peptide+formed+by+disulfide+bond+as+enhancer+for+gene+delivery&btnG=)
[#B24] Lerner, A. M., Yumerefendi, H., Goudy, O. J., Strahl, B. D., and Kuhlman, B. (2018). Engineering improved photoswitches for the control of nucleocytoplasmic distribution. *ACS Synth. Biol.* 7, 2898–2907. doi:10.1021/acssynbio.8b00368
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/30441907/) | [CrossRef Full Text](https://doi.org/10.1021/acssynbio.8b00368) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Engineering+improved+photoswitches+for+the+control+of+nucleocytoplasmic+distribution&btnG=)
[#B25] Levenson, R., Bracken, C., Bush, N., and Morse, D. E. (2016). Cyclable condensation and hierarchical assembly of metastable reflectin proteins, the drivers of tunable biophotonics. *J. Biol. Chem.* 291, 4058–4068. doi:10.1074/jbc.M115.686014
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/26719342/) | [CrossRef Full Text](https://doi.org/10.1074/jbc.M115.686014) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Cyclable+condensation+and+hierarchical+assembly+of+metastable+reflectin+proteins,+the+drivers+of+tunable+biophotonics&btnG=)
[#B26] Levenson, R., Bracken, C., Sharma, C., Santos, J., Arata, C., Malady, B., et al. (2019). Calibration between trigger and color: Neutralization of a genetically encoded coulombic switch and dynamic arrest precisely tune reflectin assembly. *J. Biol. Chem.* 294, 16804–16815. doi:10.1074/jbc.ra119.010339
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/31558609/) | [CrossRef Full Text](https://doi.org/10.1074/jbc.ra119.010339) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Calibration+between+trigger+and+color:+Neutralization+of+a+genetically+encoded+coulombic+switch+and+dynamic+arrest+precisely+tune+reflectin+assembly&btnG=)
[#B27] Levenson, R., DeMartini, D. G., and Morse, D. E. (2017). Molecular mechanism of reflectin’s tunable biophotonic control: Opportunities and limitations for new optoelectronics. *Apl. Mater* 5, 104801. doi:10.1063/1.4985758
[CrossRef Full Text](https://doi.org/10.1063/1.4985758) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Molecular+mechanism+of+reflectin%E2%80%99s+tunable+biophotonic+control:+Opportunities+and+limitations+for+new+optoelectronics&btnG=)
[#B28] Li, H., Zhang, Q., Gu, Y., Wu, Y., Wang, Y., Wang, L., et al. (2020). Efficient photoactivatable Dre recombinase for cell type-specific spatiotemporal control of genome engineering in the mouse. *Proc. Natl. Acad. Sci. U. S. A.* 117, 33426–33435. doi:10.1073/pnas.2003991117
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/33318209/) | [CrossRef Full Text](https://doi.org/10.1073/pnas.2003991117) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Efficient+photoactivatable+Dre+recombinase+for+cell+type-specific+spatiotemporal+control+of+genome+engineering+in+the+mouse&btnG=)
[#B29] Ma, L., Guan, Z., Wang, Q., Yan, X., Wang, J., Wang, Z., et al. (2020). Structural insights into the photoactivation of Arabidopsis CRY2. *Nat. Plants* 6, 1432–1438. doi:10.1038/s41477-020-00800-1
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/33199893/) | [CrossRef Full Text](https://doi.org/10.1038/s41477-020-00800-1) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Structural+insights+into+the+photoactivation+of+Arabidopsis+CRY2&btnG=)
[#B30] Mandal, D., Shirazi, A. N., and Parang, K. J. O. (2014). Self-assembly of peptides to nanostructures. *Chem. b. Self-assembly peptides nanostructures* 12, 3544–3561. doi:10.1039/c4ob00447g
[CrossRef Full Text](https://doi.org/10.1039/c4ob00447g) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Self-assembly+of+peptides+to+nanostructures&btnG=)
[#B31] Nematollahi, M. H., Torkzadeh-Mahanai, M., Pardakhty, A., Ebrahimi Meimand, H. A., and Asadikaram, G. (2018). Ternary complex of plasmid DNA with NLS-mu-mu protein and cationic niosome for biocompatible and efficient gene delivery: A comparative study with protamine and lipofectamine. *Artif. Cells Nanomed Biotechnol.* 46, 1781–1791. doi:10.1080/21691401.2017.1392316
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/29081256/) | [CrossRef Full Text](https://doi.org/10.1080/21691401.2017.1392316) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Ternary+complex+of+plasmid+DNA+with+NLS-mu-mu+protein+and+cationic+niosome+for+biocompatible+and+efficient+gene+delivery:+A+comparative+study+with+protamine+and+lipofectamine&btnG=)
[#B32] Niopek, D., Benzinger, D., Roensch, J., Draebing, T., Wehler, P., Eils, R., et al. (2014). Engineering light-inducible nuclear localization signals for precise spatiotemporal control of protein dynamics in living cells. *Nat. Commun.* 5, 4404–4411. doi:10.1038/ncomms5404
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/25019686/) | [CrossRef Full Text](https://doi.org/10.1038/ncomms5404) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Engineering+light-inducible+nuclear+localization+signals+for+precise+spatiotemporal+control+of+protein+dynamics+in+living+cells&btnG=)
[#B33] Niopek, D., Wehler, P., Roensch, J., Eils, R., and Di Ventura, B. (2016). Optogenetic control of nuclear protein export. *Nat. Commun.* 7, 10624–10629. doi:10.1038/ncomms10624
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/26853913/) | [CrossRef Full Text](https://doi.org/10.1038/ncomms10624) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Optogenetic+control+of+nuclear+protein+export&btnG=)
[#B34] Ogawa, J., Iwata, Y., Tonnu, N. U., Gopinath, C., Huang, L., Itoh, S., et al. (2020). Genetic manipulation of the optical refractive index in living cells. *bioRxiv*.
[Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Genetic+manipulation+of+the+optical+refractive+index+in+living+cells&btnG=)
[#B35] Ordinario, D. D., Phan, L., Walkup IV, W. G., Jocson, J.-M., Karshalev, E., Hüsken, N., et al. (2014). Bulk protonic conductivity in a cephalopod structural protein. *Nat. Chem.* 6, 596–602. doi:10.1038/nchem.1960
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/24950329/) | [CrossRef Full Text](https://doi.org/10.1038/nchem.1960) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Bulk+protonic+conductivity+in+a+cephalopod+structural+protein&btnG=)
[#B36] Phan, L., Kautz, R., Leung, E. M., Naughton, K. L., Van Dyke, Y., and Gorodetsky, A. A. (2016). Dynamic materials inspired by cephalopods. *Chem. Mater* 28, 6804–6816. doi:10.1021/acs.chemmater.6b01532
[CrossRef Full Text](https://doi.org/10.1021/acs.chemmater.6b01532) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Dynamic+materials+inspired+by+cephalopods&btnG=)
[#B37] Phan, L., Ordinario, D. D., Karshalev, E., Walkup IV, W. G., Shenk, M. A., and Gorodetsky, A. A. (2015). Infrared invisibility stickers inspired by cephalopods. *J. Mat. Chem. C* 3, 6493–6498. doi:10.1039/c5tc00125k
[CrossRef Full Text](https://doi.org/10.1039/c5tc00125k) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Infrared+invisibility+stickers+inspired+by+cephalopods&btnG=)
[#B38] Qin, G., Dennis, P. B., Zhang, Y., Hu, X., Bressner, J. E., Sun, Z., et al. (2013). Recombinant reflectin-based optical materials. *J. Polym. Sci. Pol. Phys.* 51, 254–264. doi:10.1002/polb.23204
[CrossRef Full Text](https://doi.org/10.1002/polb.23204) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Recombinant+reflectin-based+optical+materials&btnG=)
[#B39] Shi, T.-Q., Gao, J., Wang, W.-J., Wang, K.-F., Xu, G.-Q., Huang, H., et al. (2019). CRISPR/Cas9-based genome editing in the filamentous fungus Fusarium fujikuroi and its application in strain engineering for gibberellic acid production. *ACS Synth. Biol.* 8, 445–454. doi:10.1021/acssynbio.8b00478
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/30616338/) | [CrossRef Full Text](https://doi.org/10.1021/acssynbio.8b00478) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=CRISPR/Cas9-based+genome+editing+in+the+filamentous+fungus+Fusarium+fujikuroi+and+its+application+in+strain+engineering+for+gibberellic+acid+production&btnG=)
[#B40] Slootweg, E., Roosien, J., Spiridon, L. N., Petrescu, A.-J., Tameling, W., Joosten, M., et al. (2010). Nucleocytoplasmic distribution is required for activation of resistance by the potato NB-LRR receptor Rx1 and is balanced by its functional domains. *Plant Cell* 22, 4195–4215. doi:10.1105/tpc.110.077537
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/21177483/) | [CrossRef Full Text](https://doi.org/10.1105/tpc.110.077537) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Nucleocytoplasmic+distribution+is+required+for+activation+of+resistance+by+the+potato+NB-LRR+receptor+Rx1+and+is+balanced+by+its+functional+domains&btnG=)
[#B41] Song, J., Levenson, R., Santos, J., Velazquez, L., Zhang, F., Fygenson, D., et al. (2020). Reflectin proteins bind and reorganize synthetic phospholipid vesicles. *Langumir* 36, 2673–2682. doi:10.1021/acs.langmuir.9b03632
[CrossRef Full Text](https://doi.org/10.1021/acs.langmuir.9b03632) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Reflectin+proteins+bind+and+reorganize+synthetic+phospholipid+vesicles&btnG=)
[#B42] Song, J., Liu, C., Li, B., Liu, L., Zeng, L., Ye, Z., et al. (2022). Tunable cellular localization and extensive cytoskeleton-interplay of reflectins. *Front. Cell Dev. Biol.* 10, 862011. doi:10.3389/fcell.2022.862011
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/35813206/) | [CrossRef Full Text](https://doi.org/10.3389/fcell.2022.862011) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Tunable+cellular+localization+and+extensive+cytoskeleton-interplay+of+reflectins&btnG=)
[#B43] Szeto, H. H., Liu, S., Soong, Y., Wu, D., Darrah, S. F., Cheng, F.-Y., et al. (2011). Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. *J. Am. Soc. Nephrol.* 22, 1041–1052. doi:10.1681/asn.2010080808
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/21546574/) | [CrossRef Full Text](https://doi.org/10.1681/asn.2010080808) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Mitochondria-targeted+peptide+accelerates+ATP+recovery+and+reduces+ischemic+kidney+injury&btnG=)
[#B44] T Das, A., Tenenbaum, L., and Berkhout, B. (2016). Tet-on systems for doxycycline-inducible gene expression. *Curr. gene Ther.* 16, 156–167. doi:10.2174/1566523216666160524144041
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/27216914/) | [CrossRef Full Text](https://doi.org/10.2174/1566523216666160524144041) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Tet-on+systems+for+doxycycline-inducible+gene+expression&btnG=)
[#B45] Tammam, S. N., Azzazy, H. M., Breitinger, H. G., and Lamprecht, A. (2015). Chitosan nanoparticles for nuclear targeting: The effect of nanoparticle size and nuclear localization sequence density. *Mol. Pharm.* 12, 4277–4289. doi:10.1021/acs.molpharmaceut.5b00478
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/26465978/) | [CrossRef Full Text](https://doi.org/10.1021/acs.molpharmaceut.5b00478) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Chitosan+nanoparticles+for+nuclear+targeting:+The+effect+of+nanoparticle+size+and+nuclear+localization+sequence+density&btnG=)
[#B46] Tang, T.-C., An, B., Huang, Y., Vasikaran, S., Wang, Y., Jiang, X., et al. (2020). Materials design by synthetic biology. *Nat. Rev. Mater.* 6, 332–350. doi:10.1038/s41578-020-00265-w
[CrossRef Full Text](https://doi.org/10.1038/s41578-020-00265-w) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Materials+design+by+synthetic+biology&btnG=)
[#B47] Tao, A. R., DeMartini, D. G., Izumi, M., Sweeney, A. M., Holt, A. L., and Morse, D. E. (2010). The role of protein assembly in dynamically tunable bio-optical tissues. *Biomaterials* 31, 793–801. doi:10.1016/j.biomaterials.2009.10.038
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/19906421/) | [CrossRef Full Text](https://doi.org/10.1016/j.biomaterials.2009.10.038) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=The+role+of+protein+assembly+in+dynamically+tunable+bio-optical+tissues&btnG=)
[#B48] Vogel, P., Hanswillemenke, A., and Stafforst, T. (2017). Switching protein localization by site-directed RNA editing under control of light. *ACS Synth. Biol.* 6, 1642–1649. doi:10.1021/acssynbio.7b00113
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/28562030/) | [CrossRef Full Text](https://doi.org/10.1021/acssynbio.7b00113) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Switching+protein+localization+by+site-directed+RNA+editing+under+control+of+light&btnG=)
[#B49] Wang, H., Feng, Z., and Xu, B. (2019). Assemblies of peptides in a complex environment and their applications. *Angew. Chem.* 131, 10532–10541. doi:10.1002/ange.201814552
[CrossRef Full Text](https://doi.org/10.1002/ange.201814552) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Assemblies+of+peptides+in+a+complex+environment+and+their+applications&btnG=)
[#B50] Williams, T. L., Senft, S. L., Yeo, J., Martin-Martinez, F. J., Kuzirian, A. M., Martin, C. A., et al. (2019). Dynamic pigmentary and structural coloration within cephalopod chromatophore organs. *Nat. Commun.* 10, 1004. doi:10.1038/s41467-019-08891-x
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/30824708/) | [CrossRef Full Text](https://doi.org/10.1038/s41467-019-08891-x) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Dynamic+pigmentary+and+structural+coloration+within+cephalopod+chromatophore+organs&btnG=)
[#B51] Yang, L., Jiang, W., Qiu, L., Jiang, X., Zuo, D., Wang, D., et al. (2015). One pot synthesis of highly luminescent polyethylene glycol anchored carbon dots functionalized with a nuclear localization signal peptide for cell nucleus imaging. *Nanoscale* 7, 6104–6113. doi:10.1039/c5nr01080b
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/25773263/) | [CrossRef Full Text](https://doi.org/10.1039/c5nr01080b) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=One+pot+synthesis+of+highly+luminescent+polyethylene+glycol+anchored+carbon+dots+functionalized+with+a+nuclear+localization+signal+peptide+for+cell+nucleus+imaging&btnG=)
[#B52] Yang, L., Wang, Z., Wang, J., Jiang, W., Jiang, X., Bai, Z., et al. (2016). Doxorubicin conjugated functionalizable carbon dots for nucleus targeted delivery and enhanced therapeutic efficacy. *Nanoscale* 8, 6801–6809. doi:10.1039/c6nr00247a
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/26957191/) | [CrossRef Full Text](https://doi.org/10.1039/c6nr00247a) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Doxorubicin+conjugated+functionalizable+carbon+dots+for+nucleus+targeted+delivery+and+enhanced+therapeutic+efficacy&btnG=)
[#B53] Yu, C., Li, Y., Zhang, X., Huang, X., Malyarchuk, V., Wang, S., et al. (2014). Adaptive optoelectronic camouflage systems with designs inspired by cephalopod skins. *Proc. Natl. Acad. Sci. U. S. A.* 111, 12998–13003. doi:10.1073/pnas.1410494111
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/25136094/) | [CrossRef Full Text](https://doi.org/10.1073/pnas.1410494111) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Adaptive+optoelectronic+camouflage+systems+with+designs+inspired+by+cephalopod+skins&btnG=)
[#B54] Yu, Y., Wu, X., Guan, N., Shao, J., Li, H., Chen, Y., et al. (2020). Engineering a far-red light–activated split-Cas9 system for remote-controlled genome editing of internal organs and tumors. *Sci. Adv.* 6, eabb1777. doi:10.1126/sciadv.abb1777
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/32923591/) | [CrossRef Full Text](https://doi.org/10.1126/sciadv.abb1777) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Engineering+a+far-red+light%E2%80%93activated+split-Cas9+system+for+remote-controlled+genome+editing+of+internal+organs+and+tumors&btnG=)
[#B55] Yumerefendi, H., Dickinson, D. J., Wang, H., Zimmerman, S. P., Bear, J. E., Goldstein, B., et al. (2015). Control of protein activity and cell fate specification via light-mediated nuclear translocation. *PLoS ONE* 10, e0128443. doi:10.1371/journal.pone.0128443
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/26083500/) | [CrossRef Full Text](https://doi.org/10.1371/journal.pone.0128443) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Control+of+protein+activity+and+cell+fate+specification+via+light-mediated+nuclear+translocation&btnG=)
[#B56] Zelzer, M., and Ulijn, R. V. (2010). Next-generation peptide nanomaterials: Molecular networks, interfaces and supramolecular functionality. *Chem. Soc. Rev.* 39, 3351–3357. doi:10.1039/c0cs00035c
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/20676412/) | [CrossRef Full Text](https://doi.org/10.1039/c0cs00035c) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Next-generation+peptide+nanomaterials:+Molecular+networks,+interfaces+and+supramolecular+functionality&btnG=)
[#B57] Zhang, L., Wang, L., Xie, Y., Wang, P., Deng, S., Qin, A., et al. (2019). Triple-targeting delivery of CRISPR/Cas9 to reduce the risk of cardiovascular diseases. *Angew. Chem. Int. Ed.* 58, 12534–12538. doi:10.1002/ange.201903618
[CrossRef Full Text](https://doi.org/10.1002/ange.201903618) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Triple-targeting+delivery+of+CRISPR/Cas9+to+reduce+the+risk+of+cardiovascular+diseases&btnG=)
[#B58] Zhou, X., Vink, M., Klaver, B., Berkhout, B., and Das, A. (2006). Optimization of the Tet-On system for regulated gene expression through viral evolution. *Gene Ther.* 13, 1382–1390. doi:10.1038/sj.gt.3302780
[PubMed Abstract](https://pubmed.ncbi.nlm.nih.gov/16724096/) | [CrossRef Full Text](https://doi.org/10.1038/sj.gt.3302780) | [Google Scholar](https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Optimization+of+the+Tet-On+system+for+regulated+gene+expression+through+viral+evolution&btnG=)Keywords: synthetic peptide, RfA1, preselected and spatiotemporal tunable, cyto/nucleoplasmic location, protein transportation
Citation: Song J, Liu C, Li B, Liu L, Zeng L, Ye Z, Wu W, Zhu L and Hu B (2023) Synthetic peptides for the precise transportation of proteins of interests to selectable subcellular areas. Front. Bioeng. Biotechnol. 11:1062769. doi: 10.3389/fbioe.2023.1062769
Received: 06 October 2022; Accepted: 09 February 2023; Published: 20 February 2023.
Edited by:
Yaojun Tong, Shanghai Jiao Tong University, China
Reviewed by:
Dongsoo Yang, Korea University, Republic of Korea
Gao-Yi Tan, East China University of Science and Technology, China
Copyright © 2023 Song, Liu, Li, Liu, Zeng, Ye, Wu, Zhu and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junyi Song, c29uZ2p1bnlpQG51ZHQuZWR1LmNu; Lingyun Zhu, bGluZ3l1bnpodUBudWR0LmVkdS5jbg==; Biru Hu, aHViaXJ1MDhAbnVkdC5lZHUuY24=
†These authors have contributed equally to this work
